

Aust J Crop Sci. 19(04):398-407 (2025) | ISSN:1835-2707
https://doi.org/10.21475/ajcs.25.19.04.p266
Morphological, physiological and rice yield in lowland and upland under drought stress
Suhadi Sapto Yuwono1,3, Iskandar Lubis2*, Munif Ghulamahdi2, Endah Retno Palupi2
1Graduates Student Department of Agronomy and Horticulture, IPB University, Bogor, Indonesia
2Department of Agronomy and Horticulture, IPB University, Bogor, Indonesia
3National Agricultural Training Center of Lampung, Ministry of Agricultural, Indonesia
Corresponding author: Iskandar Lubis 
 ORCID iD : https://orcid.org/0000-0001-5326-8519
ORCID iD : https://orcid.org/0000-0001-5326-8519
Abstract: Drought is a significant factor contributing to the reduction in rice production. Therefore, this study aimed to examine the morphological, physiological, and yield responses of rice under lowland and upland planting methods subjected to drought treatment. The experiment was conducted in a greenhouse using a split-plot design with 3 replications. The main plot was water regime treatments, while the subplot was rice varieties. Water regimes included 4 levels, namely lowland planting method (T1), lowland planting method with drought treatment (T2), upland planting method (T3), and upland planting method with drought treatment (T4). Drought stress treatment was applied at 55 DAP (days after planting) by stopping watering until the plants showed a leaf rolling score of level 9 (leaf fully rolled) based on standard evaluation system for rice by IRRI. The plant materials used were 4 rice varieties, such as IPB 3S, Inpago 10, IPB 9G, and IR 64 respectively. The results showed that water regime treatments led to reduced plant height, root volume, and root weight. In upland planting method, IPB 9G produced the longest root length of 78.0 cm. Furthermore, the number of productive tillers and root length showed no significant differences under the treatment. Chlorophyll content, stomatal conductance, and intercellular CO2 levels were significantly affected by drought. Flowering age, panicle length, grains per panicle, and the weight of 100 grains were also affected. Based on observation, Inpago 10 had a faster flowering age of 90.33 DAP. The highest panicle length, grains per panicle, and rice yield per pot were produced by IPB 9G, suggesting the potential for cultivation in lowland, upland, and drought-affected environments.
Introduction
Rice is a staple food in Indonesia, with consumption reaching 22,639,224 tonnes in 2023 (BPS, 2024). This represents the highest recorded consumption in the last 5 years. To meet rising demand, maintaining and enhancing rice productivity is essential and can be achieved through improved agricultural practices as well as expanding the planting area, including the use of drylands (Rahmawati et al., 2024). Drylands, often classified as entisol, typically contain low organic matter, which limits water retention capacity (Wilujeng et al., 2015). Meanwhile, rice productivity is highly dependent on water availability, as required by almost all growth phases (Yoshida et al., 1981). Water limitations and drought for agriculture were caused by the effects of climate change (Redfern et al., 2012). Climate patterns have a direct impact on rice productivity (Fauziah., 2024). Insufficient rainfall can lead to substantial yield reductions (Wei et al., 2022). This presents a significant challenge for Indonesia in achieving food security during climate change (Ansari et al., 2023).
Drought poses a critical challenge to rice production (Kim et al., 2020), with productivity severely impacted during periods of water scarcity. It is estimated that drought accounts for approximately 50% of rice yield losses globally (Khanthavong et al., 2021). In Indonesia, rice is cultivated using 2 primary methods, namely lowland (paddy fields) and upland (dry area). While lowland cultivation relies on irrigation, upland planting depends on rainfall during the rainy season. Upland rice, sustained by rain-fed irrigation, is particularly vulnerable to shifts in rainfall patterns caused by climate change (Rane et al., 2021). The transition from rainy to dry seasons has become increasingly unpredictable, elevating drought risks in both irrigated and non-irrigated areas. Water scarcity was predicted to affect irrigated rice fields worldwide by 2025 (Wu et al., 2017).
Rice has varying water requirements at each growth stage and possesses different abilities to adapt to drought. In this context, the reproductive stage is observed to be the most impacted (Vijayaraghavareddy et al., 2020). Indonesia has 1.2 million hectares of non-irrigated land for rice (BPS., 2020), which is more severely affected by drought. Therefore, good adaptability under both dry and sufficient water is critical for maintaining production (Akter and Hassan., 2014). Developing rice varieties with good adaptability is essential for mitigating the effects of climate change on drought-prone areas. The impact of drought during the reproductive stage in lowland and upland conditions requires thorough investigation.
Drought stress are phenomena that cause physiological deviations in plants. Under this condition, rice plants show adaptive responses such as leaf rolling to reduce surface area and lowering transpiration rates to conserve water by closing stomata. These physiological reactions decrease solar radiation capture and obstruct the transfer of CO2 and O2 between plant tissue and the atmosphere, thereby reducing the photosynthesis rate and harvest yield (Rahmawati et al., 2024). Gas exchange parameters, including transpiration rate, stomatal conductance, and photosynthetic rate, are negatively impacted by drought (Serraj et al., 2011). It is important to acknowledge that the physiological effects of drought also cause reduced chlorophyll levels and stomatal conductance (Pandey & Shukla, 2015).
The morphological response of plants during drought is observable in leaf characteristics, particularly leaf rolling. Under drought stress, the ratio of root-to-shoot tends to increase. Studies showed that soil moisture was closely related to root morphology and architecture, thereby affecting rice productivity. Roots play a crucial role in rice drought resistance (Wang, 2009). Rice is typically considered as shallow-rooted (Yoshida, 1981). Lowland rice has negative implications on water uptake efficiency compared to upland rice under water-limiting conditions (Suleiman et al., 2022). Morphophopysiological effects of drought include decreased plant height and biomass. Furthermore, the changes reflect adaptive responses that influence productivity. Drought stress negatively impacts both yield and its components (Mumtaz et al., 2018).
IPB 9G and Inpago 10 are upland rice varieties known for high productivity and potential drought resistance, with yields of 9.09 tons/ha and 7.03 tons/ha, respectively. These varieties show promise for enhancing rice production in drylands. IPB 3S, a high-productive lowland rice variety, has not been tested for drought resistance. In this study, IR 64 which is a drought-sensitive variety, was used as a control. Morphological and Physiological responses of these varieties, as well as rice yields, are essential for advancing rice breeding programs. Therefore, this study aimed to investigate the morphological and physiological responses of rice planted in lowland and upland induced by drought during the reproductive phase.
Result and Discussion
Leaf rolling score
The results showed that the leaves began to curl on the third day after water was withheld, as presented in Figure 1. Based on observation, leaves in the T4 treatment curled faster than the T2 treatment. Furthermore, this difference was attributed to the distinct initial water condition. T4 was upland water treatment, while T2 represented lowland rice cultivation, which maintained higher soil moisture content. Water drying treatment commenced 55 days after planting, corresponding to the reproductive stage of the plants. T4 showed the earliest signs of leaf rolling, as upland planting conditions store water only at field capacity. When irrigation stopped, soil moisture rapidly depleted, leading to a lack of water for the plant. Among the varieties tested, IR 64 had the highest leaf rolling score at 8 days after treatments, signifying its sensitivity to drought. IPB 3S was also identified as susceptible, while IPB 9G and Inpago 10 showed moderate tolerance to drought. Leaf rolling score is used to investigate rice resistance to drought (Fauziah et al. 2024). Under water stress, plants respond through various adaptations, such as changes in photosynthetic rates, stomatal closure, and leaf-rolling mechanisms (Panda et al., 2021). Leaf rolling is recognized as an early response that either precedes or follows stomatal closure. Additionally, it serves to lower the transpiration rate by limiting the leaf surface exposed to sunlight.
Differences in rice morphology due to drought stress
The results showed that water treatment influenced plant height for all varieties. Both water treatment and variety had a statistically significant effect on plant height, as presented in Table 4. Reduction in height was observed between T1 to T2, T3 to T4, and T1 to T3. The percentage of decrease from T1 to T2 was 2%, 1 %, 1%, and -2% for IPB 3S, inpago 10, IPB 9G, and IR 64, respectively. From T3 to T4 the reduction was 2%, 1%, 1 %, and 4% for IPB 3 S, Inpago 10, IPB 9G, and IR 64, respectively. The highest decrease in plant height occurred between T1 to T3, with reductions of 7.55%, 15.33%, 14.41%, and 10.84% for IPB 3S, Inpago 10, IPB 9G, and IR 64, respectively.
Drought stress was introduced by stopping irrigation at the onset of the reproductive stage (55 DAP) and continued until a treatment reached a leaf rolling score of 9 (IRRI., 2002). The condition of water scarcity is a critical abiotic stress that adversely affects plant growth and development (Suralta & Inukai, 2014). The lack of water during drought inhibited Plant growth, with height in T1 being significantly (P <0.05) higher than other treatments. In line with previous studies, flooding conditions produced taller plants than non-flooding conditions (Patel et al., 2010). Plant height has proven to be highly sensitive to water limitations (Vijayaraghavareddy et al., 2020). It was important to acknowledge that plant growth was related to yield, as enhanced shoot growth and greater biomass contributed to higher grain production (Zhang et al., 2009). Furthermore, drought stress was shown to decrease the dry weight of plant biomass in all rice cultivars (Miftahudin et al. 2020). This suggests that plant height significantly influences both biomass and yield. No significant differences in plant height were detected between T1 and T2, as well as T3 and T4. Drought stress T2 and T4 were imposed when maximum height was almost attained, hence, height loss is minimized compared to T1 and T3.
Decreased soil water content during the tillering stage led to a reduction in the growth of total productive tillers. It was important to acknowledge that drought had a significant effect on all rice varieties. IR 64 in T1 treatment produced the highest number of productive
Table 1. Description of rice seed materials.
| Varieties | Types | Origin | Descriptions |
|---|---|---|---|
| IPB 3S | Lowland rice | Indonesia | High yield lowland rice |
| IPB 9G | Upland rice | Indonesia | High yield upland rice |
| Inpago 10 | Upland rice | Indonesia | High yield upland rice |
| IR 64 | Lowland rice | Phillipines | Lowland rice, susceptible to drought |
Table 2. Temperatures and humidity on study area.
| Temperature/RH | August | September | October | November | December |
|---|---|---|---|---|---|
| Highest temperature | 35.1°C | 40.1 °C | 40.9 °C | 43.6 °C | 44.0 °C |
| Mean | 26.4 °C | 24.7 °C | 28.9 °C | 28.9 °C | 29.3 °C |
| Lowest temperature | 22.0 °C | 22.2 °C | 21.6 °C | 22.6 °C | 19.5 °C |
| Relative humidity | 61.5% | 55.4% | 67.8 % | 62.2% | 63.8% |
Table 3. Leaf rolling score (IRRI, 2002).
| Score | Symptoms | Category |
|---|---|---|
| 0 | Healthy leaves | Highly tolerant |
| 1 | Leaves starting to curl (shallow) | Tolerant |
| 3 | Leaves curl (deep V shape) | Moderate tolerant |
| 5 | Leaves curl (curved U shape) | Moderate susceptible |
| 7 | Leaves curl where leaf edges touch (O shape) | Susceptible |
| 9 | Leaves fully rolled | Very susceptible |
Tabel 4. Leaf rolling result in lowland upland method planting by leaf rolling score.
| Variety | Treatment | Drought treatments | Score | Category |
|---|---|---|---|---|
| IPB 3S | T2 | 8 days | 3 | Moderate tolerant |
| Inpago 10 | T2 | 8 days | 3 | Moderate tolerant |
| IPB 9G | T2 | 8 days | 1 | Tolerant |
| IR 64 | T2 | 8 days | 5 | Moderate susceptible |
| IPB 3S | T4 | 8 days | 7 | Susceptible |
| Inpago 10 | T4 | 8 days | 3 | Moderate tolerant |
| IPB 9G | T4 | 8 days | 3 | Moderate tolerant |
| IR 64 | T4 | 8 days | 9 | Very susceptible |
tillers compared to other varieties. It had 25.33 tillers, while Inpago, IPB 3S, and IPB 9G, 10 18.83, 14.00, and 13.95 tillers, respectively. In this study, T1 treatments produced more tillers than T3 treatments. No significant treatments were observed among IPB 3S, Inpago 10, and IPB 9G when comparing T1 with T2, T3, and T4. This implied that drought and dryland conditions did not affect on total productive tiller of the varieties. In contrast, IR 64 experienced a significant reduction in productive tillers from T1 to T3 and T4. Inpago 10 and IPB 9G, as upland varieties, showed relative drought tolerance, while IR 64, lowland variety, was sensitive to drought. IPB 3S, categorized as lowland rice plant, had a lower total tiller count. The number of tillers had a close and direct correlation with yield. In line with the study by (Xu et al., 2020), drought stress in the tillering phase decreased rice yield. Rahmawati et al., (2024) stated that high-level drought significantly affected plant height during both the vegetative and mature stages. The water scarcity condition also had a negative impact on the number of tillers and productive tillers.
Changes in root length due to water treatment did not show significant differences across varieties, as presented in Table 4. However, rice variety had a significant effect on root length. IPB 9G produced the longest roots of 88 cm in T3 compared to other varieties in T1, T2, and T4 treatments, as shown in Figure 2. The anatomical structure of upland rice roots showed water-saving and drought-resistant properties (Zhang et al., 2020). As upland variety, IPB 9G is well suited for producing longer roots, a key characteristic of drought-tolerant rice (Mayly et al., 2015). Panda et al., (2021) stated that long roots play a vital role in avoiding drought stress. According toAdeboye et al., (2021), root parameters, including root length, contribute to drought adaptation mechanism. According to Sikuku et al., (2010), root length reduced with a decrease in water availability.
Water treatment showed a significant effect (P <0.05) on root volume, as presented in Table 4. T1 and T2 treatments produced higher root volume than T3 and T4. Based on the DMRT test, T1 and T2 were not significantly different, implying that drought conditions on lowland planting method had no substantial impact. Following previous studies, root volume and biomass were higher in flooded than in aerobic conditions (Patel et al., 2010).
Root weight, a crucial factor in plant growth, had significant differences across treatments. Based on observation, T1 produced a higher root weight than T3. Aziez et al., (2018) stated that intermittent irrigation increased dry root weight in the Mentik Wangi variety. This study did not observe an increase in the parameter under drought conditions. T1 also produced higher root weights than T2 and T4. Among the varieties, IPB 9G recorded the highest across all treatments. The root
Figure 1. A. lowland treatment B. upland treatment C. lowland with drought D. Upland with drought.
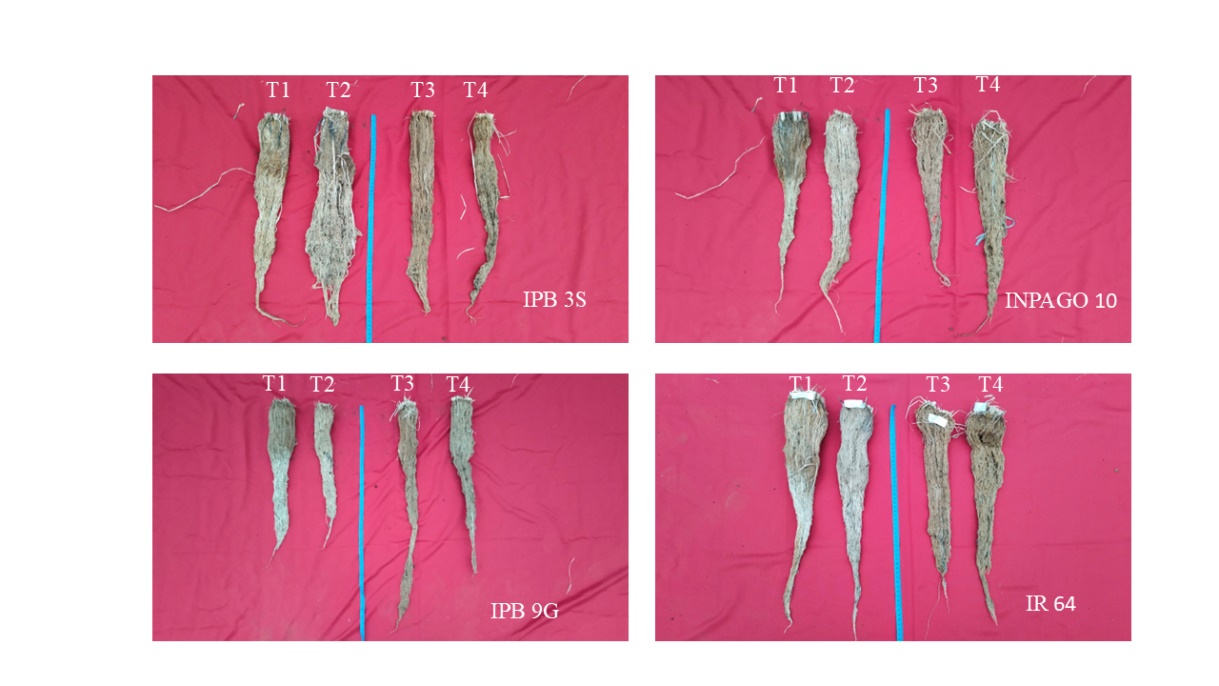
Figure 2. Root length of rice varieties due to water treatments: T1= lowland T2 = lowland with drought at reproductive stage, T3 = upland, and T4 = upland with drought at reproductive stage.
system plays a vital role in enhancing resistance to water shortages (Raumjit et al., 2019). Longer roots with high volume were needed for plant resistance in different environments. Suralta et al., (2018) stated that the development of the root system contributed to increased water and nutrient uptake from the soil. Furthermore, differences in root characteristics between lowland and upland varieties were observed by (Sandar et al., 2022). Increasing rice yields can be achieved by fostering grain production through enhanced root activity during the grain-filling stage (Zhang et al., 2009).
Differences in plant physiology due to drought stress
Chlorophyll serves as a central component of photochemical reactions in leaf tissue. Its content changed due to chloroplast damage caused by reactive oxygen species (ROS) regeneration during water shortages. Based on the results in Table 5, total chlorophyll levels were influenced by water treatment factors. Among the treatments, T1, T2, T3, and T4 had the highest total chlorophyll content in IPB 3S, IR 64, IR 64, and Inpago 10, respectively. According to previous studies, drought conditions caused changes in chlorophyll content due to damage on chloroplasts and reactive
oxygen species regeneration during the drying process (Salsinha et al., 2020). Results showed that the content reduced as drought occurred.
The photosynthetic rate was influenced by rice variety, while stomatal conductance and intercellular CO₂ concentration were affected by water treatments, as presented in Table 5. However, the transpiration rate was not influenced by either water treatments or varieties. The highest and lowest photosynthetic rates were recorded for IPB 9G and IR 64, respectively, under T2 and T4. The highest stomatal conductance and CO2 intercellular was observed in IPB 9G under T1. Photosynthesis, a critical metabolic process for plant growth, is affected by water deficit or drought stress (Panda et al., 2021). Previous studies have shown that gas exchange parameters such as stomatal conductance decreased with increasing stress in all rice genotypes at different cropping stages (Vijayaraghavareddy et al., 2020). Drought limited photosynthesis primarily through stomatal closure, reduced carbon dioxide diffusion, decreased photosynthetic enzyme activity, and reduced efficiency of photosystem II (Salsinha et al., 2020). This decline in photosynthesis due to stress at each growth phase, directly impacted yield (Vijayaraghavareddy et al.,
Table 5. Morphology of rice varieties due to water treatments.
| Treatments | Varieties | Plant height (cm) | Tillers | Root length (cm) | Root volume (ml) | Root weight (g) |
|---|---|---|---|---|---|---|
| T1 | IPB 3S | 154.9 ab | 14.00 e | 61.0 bc | 283.3 abc | 43.01 ab |
| Inpago 10 | 156.6 ab | 18.83 cd | 59.6 bc | 325.0 a | 52.84 ab | |
| IPB 9G | 164.5 a | 13.95 e | 57.3 bc | 316.6 ab | 56.68 a | |
| IR 64 | 137.5 d | 25.33 a | 49.6 c | 366.6 a | 50.70 ab | |
| T2 | IPB 3S | 151.8 bc | 13.86 e | 69.3 ab | 341.6 a | 41.56 ab |
| Inpago 10 | 155.7 ab | 19.25 cd | 62.0 bc | 308.3 ab | 33.61 b | |
| IPB 9G | 163.4 ab | 12.91 e | 60.3 bc | 383.3 a | 45.26 ab | |
| IR 64 | 139.9 d | 23.66 ab | 54.0 bc | 383.3 a | 44.63 ab | |
| T3 | IPB 3S | 143.2 cd | 12.50 e | 57.3 bc | 158.3 cd | 35.75 b |
| Inpago 10 | 132.6 de | 17.47 d | 52.3 bc | 166.6 cd | 39.52 b | |
| IPB 9G | 140.8 cd | 13.47 e | 78.0 a | 100.0 d | 34.96 b | |
| IR 64 | 122.6 ef | 21.55 bc | 57.0 bc | 191.6 bcd | 39.37 ab | |
| T4 | IPB 3S | 140.7 cd | 12.00 e | 62.3 bc | 101.6 d | 34.07 b |
| Inpago 10 | 131.9 de | 17.23 d | 64.6 abc | 108.3 d | 34.37 b | |
| IPB 9G | 138.1 d | 13.27 e | 70.3 ab | 125 d | 36.25 b | |
| IR 64 | 117.8 f | 21.36 bc | 58.0 bc | 116.6 d | 39.75.ab | |
| Treatment (T) | ** | ns | ns | ** | ** | |
| Varieties (V) | ** | ** | * | ns | ns | |
| T*V | ns | ns | ns | ns | ns |
Note: *and ** F values are significant at 0.05 and 0.01 probability levels, respectively. Ns means non-significant at the P = 0.05 level. Different letters show statistical significance at the P = 0.05 level within the same column based on the DMRT test. T1= lowland T2 = lowland with drought at reproductive stage, T3 = upland, and T4 = upland with drought at reproductive stage. T=treatment, V=varieties
Table 6. Physiology of rice varieties due to water treatments.
| Treatment | Varieties | Chlorophyll total | Photosynthetic rate (µmol m-2s-1) | Stomatal Conductance (mol m⁻² s⁻¹) | CO2 Inter Cellular (µmol mol-1) | Transpiration rate (mol m⁻² s⁻¹) |
|---|---|---|---|---|---|---|
| T1 | IPB 3S | 4.30 ab | 21.83 a | 0.1414 ab | 117.63 abc | 2.21 a |
| Inpago 10 | 3.85 bc | 21.28 abc | 0.1465 ab | 136.09 ab | 2.07 a | |
| IPB 9G | 3.80 bc | 21.99 a | 0.1658 a | 157.61 a | 2.11 a | |
| IR 64 | 3.90 bc | 19.53 abcd | 0.1421 ab | 153.14 ab | 2.18 a | |
| T2 | IPB 3S | 3.84 bc | 21.96 a | 0.1641 ab | 154.72 ab | 2.07 a |
| Inpago 10 | 3.66 bc | 21.99 a | 0.1536 ab | 140.23 ab | 2.09 a | |
| IPB 9G | 3.21 c | 22.14 a | 0.1522 ab | 132.94 ab | 2.08 a | |
| IR 64 | 4.75 a | 19.56 abcd | 0.1460 ab | 136.85ab | 1.67 b | |
| T3 | IPB 3S | 2.98 c | 22.07 a | 0.1402 ab | 105.18 abc | 2.23 a |
| Inpago 10 | 3.20 c | 19.01 bcd | 0.1280 ab | 133.22 ab | 2.28 a | |
| IPB 9G | 3.23 c | 20.71 abcd | 0.1322 ab | 108.38 abc | 2.27 a | |
| IR 64 | 3.66 bc | 18.71 cd | 0.1208 ab | 107.03 abc | 2.08 a | |
| T4 | IPB 3S | 3.20 c | 21.61 ab | 0.1208 ab | 76.53 c | 2.07 a |
| Inpago 10 | 3.31 c | 18.85 cd | 0.1267 ab | 121.70 abc | 2.03 a | |
| IPB 9G | 3.00 c | 21.32 abc | 0.1371 ab | 101.28 bc | 2.32 a | |
| IR 64 | 3.12 c | 18.12 d | 0.1219 b | 120.67 abc | 1.97 ab | |
| T | * | ns | *** | *** | ns | |
| V | ns | *** | ns | ns | ns | |
| T*V | ns | ns | ns | ns | ns |
Note: *and ** F values are significant at 0.05 and 0.01 probability levels, respectively. Ns means non-significant at the P = 0.05 level. Different letters show statistical significance at the P = 0.05 level within the same column based on the DMRT test. T1= lowland T2 = lowland with drought at reproductive stage, T3 = upland, and T4 = upland with drought at reproductive stage. T=treatment, V=varieties
2020). A report explained that the rate of photosynthesis and water use efficiency were much higher under aerobic conditions. In this study, a decreasing trend in stomatal conductance under T3 and T4 was observed. (Darmadi et al., 2019) stated that differences in rice types caused variations in physiological characteristics, such as water
use and efficiency. In line with Panda et al., (2021), maintaining high photosynthesis and stomatal conductance was a crucial mechanism of plant tolerance to drought.
A different trend was observed in the transpiration parameter. In this study, there was no significant
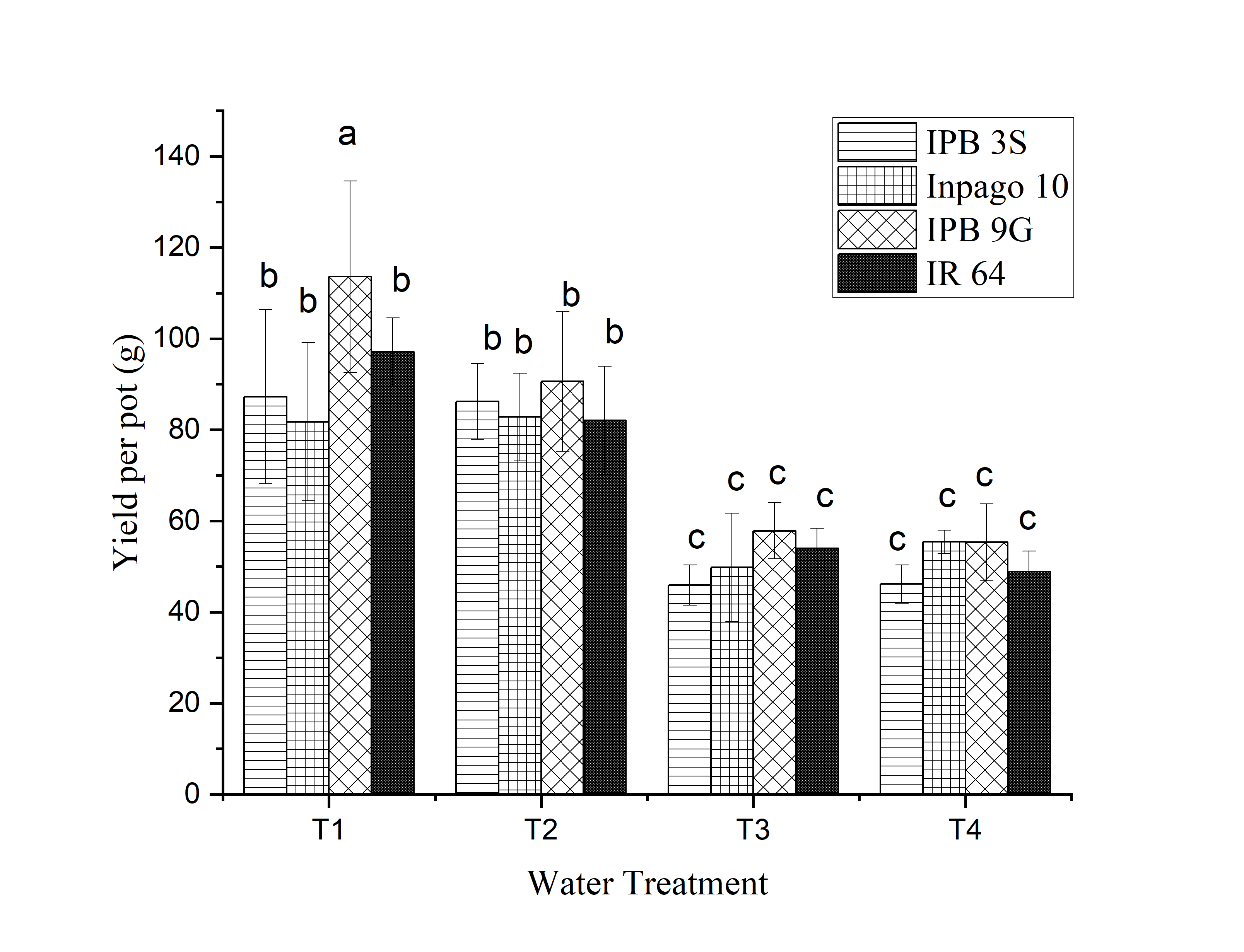
Figure 3. Yield of rice varieties due to water treatment: T1= lowland T2 = lowland with drought at reproductive stage, T3 = upland, and T4 = upland with drought at reproductive stage.
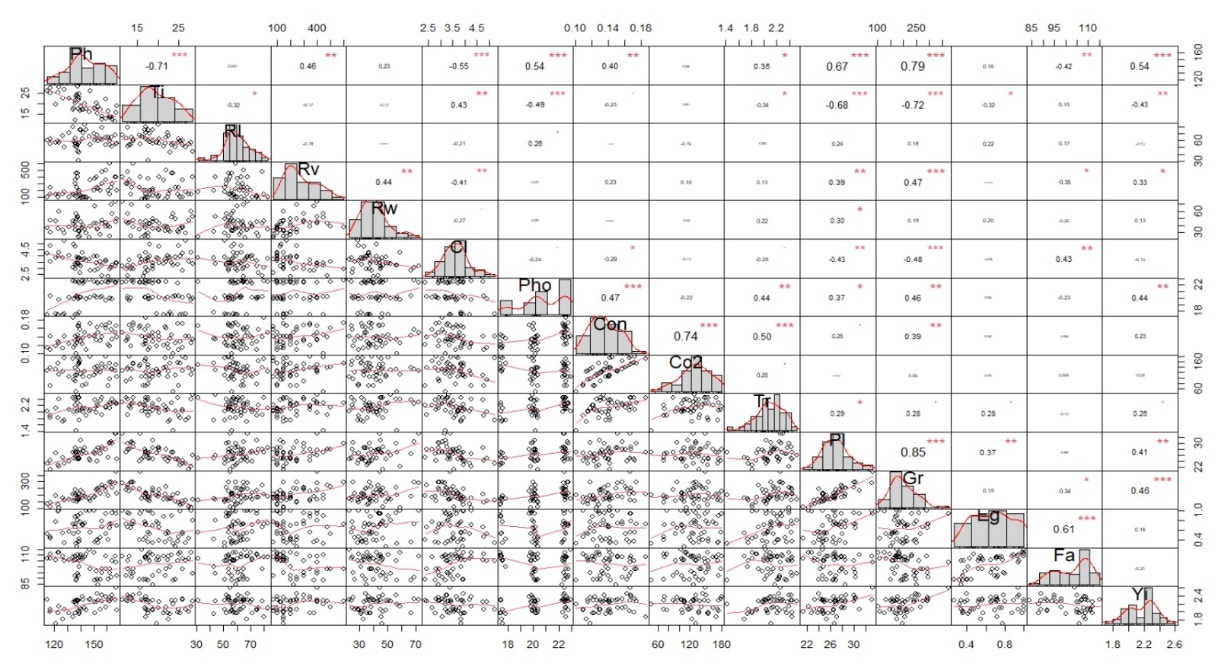
Figure 4. Correlation between yield, morphological and physiological parameters. Ph: plant height, Ti: number of tiller, Rl: root length, Rv: root volume, Rw: root weight, Cl:Chlorophyll content, Pho: photosynthetic rates, Con: stomatal donductances, Co2: CO2 inter cellular, Tr: Transpiration rates, Pl: panicle legth, Gr:number grains per panicles, Eg: empty grains, Fa: flowering age and Yi: yield per pot.
difference in both water and variety factors. A low transpiration rate is needed to increase crop yields, as observed in IR 64. This is in line with the study that cultivars with higher transpiration rates had higher dry matter production (Hien et al. 2023). The higher tiller production in IR 64 may contribute to increased dry matter. Contrasting results from Sandar et al., (2022) stated that photosynthesis rate, stomatal conductance, and transpiration rate negatively influenced biomass in lowland and upland rice. Drought stress negatively impacts gas exchange parameters, including photosynthetic rate, transpiration rate, and stomatal conductance, respectively (Mumtaz et al., 2020). Based on Patel et.al., (2010) reported higher stomatal conductance under flooding conditions, with no significant difference in transpiration rate between the two water management systems. Meanwhile, Sikuku et al., (2010) stated that drought-stressed plants had lower transpiration and stomatal conductance rates compared to to well-watered plants across all the varieties. The decreased trend in
physiological traits occurred in photosynthesis, stomatal conductance, and CO2 intercellular, from T1, T2, T3, and T4, respectively.
Effect of drought stress on rice yield and yield components
The results showed that flowering age, panicle length, and grain per panicle were influenced by water and a variety of treatments, as presented in Table 7. T3 and T4 treatments extended the flowering age, leading to delayed flowering than T1 and T2. However, there was no significant difference in flowering time between T1 and T2 or between T3 and T4. Among the varieties, Inpago 10 had the fastest flowering time. This is in line with the study that drought treatments imposed a delay on the flowering and harvesting age (Rahmawati et al., 2024).
T3 and T4 treatments led to shorter panicle lengths compared to T1 and T2. The longest panicles and most grains per panicle were produced by IPB 9G under T1. Varieties affected the number of empty grains per panicle and the weight of 100 grains. Among the varieties, Inpago
Table 7. Rice yield and yield components due to water treatments.
| Treatments | Varieties | Flowering age (DAP) | Panicle length (cm) | Grain per panicle | Empty grain (%) | 100 grain’s weight |
|---|---|---|---|---|---|---|
| T1 | IPB 3S | 96.66 bcde | 26.70 cd | 223.07 cde | 0.69 abcde | 2.33 b |
| Inpago 10 | 92.00 de | 26.24 cd | 214.21 def | 0.48 de | 2.10 d | |
| IPB 9G | 100.00 bcd | 31.14 a | 310.20 a | 0.87 a | 2.51 a | |
| IR 64 | 97.66 bcde | 25.89 cd | 162.90 ghi | 0.65 abcde | 2.27 bc | |
| T2 | IPB 3S | 94.00 cde | 26.81 cd | 258.13 bc | 0.62 abcde | 2.34 b |
| Inpago 10 | 90.33 e | 27.67 bc | 242.54 cd | 0.44 e | 2.09 d | |
| IPB 9G | 100.66 abc | 31.08 a | 293.40 ab | 0.79 abc | 2.28 bc | |
| IR 64 | 98.33 bcde | 25.61 cd | 174.17 fgh | 0.59 abcde | 2.14 cd | |
| T3 | IPB 3S | 108.66 a | 26.35 cd | 187.13 efg | 0.76 abcd | 2.26 bc |
| Inpago 10 | 103.66 ab | 25.47 cde | 194.63 efg | 0.52 bcde | 1.82 e | |
| IPB 9G | 108.66 a | 29.08 ab | 201.97 defg | 0.82 ab | 2.08 d | |
| IR 64 | 109.33 a | 23.24 ef | 135.57 hi | 0.60 abcde | 2.10 d | |
| T4 | IPB 3S | 109.33 a | 25.39 cde | 169.40 fgh | 0.74 abcd | 2.31 b |
| Inpago 10 | 102.33 abc | 24.65 def | 183.13 efg | 0.49 cde | 1.99 d | |
| IPB 9G | 108.33 a | 27.38 bc | 197.83 efg | 0.79 abc | 2.06 d | |
| IR 64 | 108.66 a | 22.49 f | 123.07 1 | 0.65 abcde | 2.15 cd | |
| T | * | * | ** | tn | * | |
| V | ** | ** | ** | ** | ** | |
| T*V | tn | tn | tn | tn | ** | |
Note: *and ** F values are significant at 0.05 and 0.01 probability levels, respectively. Ns means non-significant at the P = 0.05 level. Different letters show statistical significance at the P = 0.05 level within the same column based on the DMRT test. T1= lowland T2 = lowland with drought at reproductive stage, T3 = upland, and T4 = upland with drought at reproductive stage. T=treatment, V=varieties
10 had the smallest percentage of empty grains, while IPB 9G recorded the highest value for the 100-grain weight parameters. High grain yield was often attributed to the larger sink size (number of grains) and the larger panicle (Zhang et al., 2009). In this study, IPB 9G produced the highest number of grains. However, the number of empty grains was also the highest. Super rice varieties with high grain count per panicle had a lower percentage of filled grains than the control (Zhang et al., 2009). The larger sink size led to more assimilates from shoots being channeled to the sink during the grain-filling phase (Zhang et al., 2009).
T3 and T4 treatments produced lower productivity than T1 and T2, as shown in Figure 3. Following previous investigations, cultivating rice under aerobic conditions led to a 27.5% decrease in yield compared to flooded conditions (Patel et al., 2010). In this study, IPB 9G produced the highest yield per pot, which corresponded to the performance in terms of the highest number of grains per panicle. A greater number of grains per panicle contributed more to increasing yield and served as a key factor in differentiating aerobic and flooded rice systems (Patel et al., 2010).
The decrease In yield under water ”tres’ conditions was caused by a reduction in the number of grains per panicle and the weight of 1000 grains (Zheng et al., 2010). The results of this study showed that the weight of 100 grains in T3 and T4 was lower than in T1 and T2. Among the varieties tested, IPB 9G had the best yield per pot across all treatments. Previous studies showed that IPB 9G rice had the advantage of high water use efficiency (Darmadi et al., 2019). It effectively used water under dry conditions, while also featuring a relatively high photosynthetic capacity. These traits present a potential
for stabilizing rice yield in both lowland and upland environments, as well as under drought conditions. Breeding programs targeting high photosynthesis capacity and increased number of grains were essential for developing varieties that can adapt to climate change (Zhang et al., 2022).
Correlation between yield, morphological, and physiological parameters
Correlation between yield with morphological and physiological parameters is presented in Figure 4. Plant height, root volume, photosynthetic rate, panicle length, and number of grains per panicle were positively correlated with yield. These traits collectively contributed to increased productivity. The number of tillers showed negatively correlated with yield. Flowering age positively correlated with empty grains, while panicle length correlated with the number of grains per panicle. Drought conditions negatively impacted plant height (Sikuku et al., 2010), which then affected rice yield. According to Miftahudin et al., (2010), drought stress decreased dry weight and plant height. This is in line with a report that the decrease in plant height for IPB 9G under drought conditions was minimal, enabling the variety to sustain high yield. The tolerant lines maintained plant biomass during the reproductive stage under drought conditions (Kumar et al., 2015). Flowering stage drought had a strong correlation with yield and physiological traits in rice (Yang et.al., 2019)
Materials and Methods
Study location
The study was conducted from August 2023 to December 2023 at the Cikabayan Experiment Garden, IPB University, located in Dramaga, Bogor, West Java, Indonesia. The geographical coordinate of the Cikabayan Experimental Garden was 6°33'5.55" S and 106°42'55.35" E, with an altitude of 154 m above sea level (asl).
Plant materials
The materials used were 4 rice varieties, comprising IPB 9G and Inpago 10 (upland varieties) as well as IPB 3S and IR 64 (lowland varieties). It is important to acknowledge that IPB 9G, Inpago 10, and IPB 3S originated from Indonesia. IPB 9G and Inpago 10 were known to produce high yield in upland conditions, while IPB 3S had the best yield in lowland. IR 64, a control variety susceptible to drought, originates from the Philippines. The soil used in this study had a pH of 5.26 (H2O) and 4.55 (KCl) and contained 2.20% organic carbon, 0.25% total N, 170.61 ppm P2O5 (Bray I) of available phosphorus, and 0.96 mol k/kg of potassium. The greenhouse temperature and humidity conditions are summarized in Table 2.
Experimental design
A split-plot design with 3 replications was used for the experiment. Each replication included 12 pots with dimensions of 30 cm diameter and 40 cm height. The main plots were water treatments, while the subplots were rice varieties. The main plot treatments included lowland planting method (T1), lowland planting method with drought treatment (T2), upland planting method (T3), and upland planting method with drought treatment (T4). The subplot consists of 4 rice varieties, namely IPB 3S, Inpago 10, IPB 9G, and IR 64.
Procedure
Pots were filled with air-dried soil, with water and drought treatments applied as follows:
T1 (lowland planting method). A continuous water flooding treatment was applied, maintaining standing water approximately 3 to 5 cm above the soil surface. Water was kept stagnant from the time rice was planted until harvest.
T2 (lowland planting method with drought at reproductive stage). Standing water, approximately 3 to 5 cm above the soil surface, was maintained from planting to 55 DAP. Drought stress was then imposed by removing water and leaving the pot dry until rice leaves showed curling at level 9 (table 3). Finally, the pots were watered until harvest time.
T3 (upland planting method). The soil was sufficiently watered approximately on field capacity from planting to harvest.
T4 (upland planting method with drought stress at the reproductive stage). The treatment included watering the soil sufficiently approximately on field capacity from planting to 55 DAP. The process ceased until the plant leaves showed rolling at level 9 on the score scale (IRRI, 2002). Finally, the pots were watered after this period and maintained until harvest.
Rice seeds used were 19 days old, with 2 planted per pot for T1 and T2. In terms of T3 and T4, 5 seeds were planted directly in each pot. Rice was fertilized with 12.5 g, Urea, 0.5 g KCl, and 0.5 g SP36 per pot, equivalent to 250 kg ha-1 Urea, 100 kg ha-1 KCl, and 100 kg ha-1 SP36. All SP 36 and KCl fertilizers, alongside ½ urea, were administered 1 week after planting. The remaining urea was administered 4 weeks after planting. Before planting, the medium was treated with carbofuran insecticide. Pest and disease were controlled using insecticides and fungicides based on the observed infestation.
Measurements
Measurements were conducted on the morphological variables of rice plants, including plant height, number of tillers, root length, root volume, and root weight. Plant height was measured from the base of the stem to the highest plant crown at the end of the flowering stage. Productive tillers were determined as the number of rice tillers in each clump that developed panicles. Root measurements, including length, volume, and dry weight, were conducted 11 days after drought treatment. For these measurements, a pot from each treatment was selected. Root length and volume were determined immediately, while the dry weight was measured after drying in an oven at 80 C for 72 hours.
Yield parameters include flowering age, panicle length, grains per panicle, empty grain, the weight of 100 grains, and yield per pot. Flowering age was calculated when 50 percent of the plants in a treatment developed flowers. Meanwhile, panicle length, grains per panicle, empty grain, and the weight of 100 grains were measured at harvest.
Physiological measurements were photosynthesis rate, transpiration rate, stomatal conductance, and intercellular CO2. These were recorded using the LICOR 6400 XT at 10:00 AM, 11–12 days after drought treatments.
Leaf chlorophyll content (mg g-1) was determined using a spectrophotometer, following the method described by (Sims and Gamon 2002). A fresh leaf sample weighing 0.02 g was ground to a fine consistency using a mortar. Subsequently, 2 ml of acetone-tris (Acetris) was added to the ground sample. The mixture was transferred to a cuvette, and more Acetris was added to bring the volume to 2 ml. The solution was centrifuged for approximately 10 seconds, and 1 ml of the supernatant was extracted and added with 3 ml of Acetris. Furthermore, the mixed solution was analyzed using a spectrophotometer at wavelengths of 470 nm, 537 nm, 647 nm, and 663 nm. Chlorophyll a and b concentrations were calculated using the following formula:
\(Chlorophyll\ a = \frac{((0.01373\ x\ \lambda 663) - \ \left( 0.000897\ x\ \lambda 537) - (0.003046\ x\ \lambda 647) \right)}{sample\ weight}x8\)
\(Chlorophyll\ b = \frac{((0.012405\ x\ \lambda 647) - \ \left( 0.0004305\ x\ \lambda 537) - (0.005507\ x\ \lambda 647) \right)}{sample\ weight}x8\)
\[Clorophyll\ total = chlorophyll\ a + chlorophyll\ b\]
Data analysis
The data were analyzed using analysis of variance and continued with the Duncan Multiple Range Test using R Studio software.
Conclusion
In conclusion, lowland and upland planting conditions caused differences in the morphophysiology and yield of rice. Drought stress shortened plant height as well as decreased root volume and root weight. The number of productive tillers and root length showed no significant differences under drought treatment compared to lowland and upland planting methods. Chlorophyll content, stomatal conductances, and CO2 intercellular were significantly influenced by drought treatment. Key yield parameters, including flowering age, panicle length, number of grains per panicle, and 100-grain weight were also affected. Compared to lowland planting method, upland planting method was more adversely impacted. Among the varieties tested, IPB 9G produced the highest panicle length, number of grains per panicle, and rice yield per pot. The results suggested that IPB 9G had significant potential for cultivation across lowland, upland, and drought-affected environments.
Reference
Adeboye KA, Oduwaye OA, Daniel IO, Fofana M, Semon M (2021) Characterization of flowering time response among recombinant inbred lines of WAB638-1/PRIMAVERA rice under reproductive stage drought stress. Plant Genet Resour Characterisation Util. 19(1):1–8. doi:10.1017/S1479262121000010.
Akter A, Hassan M J (2014) AMMI biplot analysis for stability of grain yield in hybrid rice (Oryza sativa L.). Rice Res Open Access. 2(2). doi:10.4172/jrr.1000126.
Ansari A, Pranesti A, Telaumbanua M, Alam T, Taryono, Wulandari RA (2023) Evaluating the effect of climate change on rice production in Indonesia using multimodelling approach. Heliyon. 9(9):e19639. Available from: https://doi.org/10.1016/j.heliyon.2023.e19639
Aziez A., Hanudin E, Harieni S (2018) Impact of water management on root morphology, growth and yield component of lowland rice varieties under the organic system of rice intensification. J Degrad Min Lands Manag. 5(2):1035–1045. doi:10.15243/jdmlm.2018.052.1035.
Badan Litbang Pertanian (2008) Petunjuk Teknis Lapang Pengelolaan Tanaman Terpadu (PTT) Padi Gogo. Pedoman Bagi Penyuluh Pertanian. Badan Penelitian dan Pengembangan Pertanian, Jakarta.
Badan Pusat Statistik Indonesia (2024) Luas Panen dan Produksi Padi di Indonesia 2023 (Angka Tetap). https://www.bps.go.id/id/pressrelease/2024/03/01/2375/pada-2023--luas-panen-padi-mencapai-sekitar-10-21-juta-hektare-dengan-produksi-padi-sebesar-53-98-juta-ton-gabah-kering-giling--gkg-.html
Darmadi D, Junaedi A, Sopandie D, Supijatno, Lubis I, Homma K, Hidayati N (2019) Evaluation of water-saving rice status based on morphophysiological characteristics and water use efficiency. Biodiversitas. 20(10):2815–2823. doi:10.13057/biodiv/d201006.
Fauziah QN, Purwanto E, Rahayu M (2024) Phylogenetic relationship of local rice from Central Java, Indonesia with Pokkali variety based on Single Nucleotide Polymorphism (SNP) markers. Biodiversitas. 25(10):3965–3973. doi:10.13057/biodiv/d251056.
Hien NTTH, Yamanaka H, Kobata T, Hirai Y, Saitoh K (2023) Effects of water use efficiency on plant dry matter in NERICA and Japanese rice cultivars under drought conditions. IOP Conf Ser Earth Environ Sci. 1155(1). doi:10.1088/1755-1315/1155/1/012004.
IRRI (2002). Standard Evaluation System for Rice. Los Banos, IRRI
Khanthavong P, Yabuta S, Asai H, Hossain MA, Akagi I, Sakagami JI (2021) Root response to soil water status via interaction of crop genotype and environment. Agronomy. 11(4):1–15. doi:10.3390/agronomy11040708.
Kim Y, Chung YS, Lee E, Tripathi P, Heo S, Kim KH (2020) Root response to drought stress in rice (oryza sativa L.). Int J Mol Sci. 21(4):12–14. doi:10.3390/ijms21041513.
Mayly S, Rauf A, Hanum C, Hanum H (2015) Root s Bioassay of Upland Rice Varieties on Several Soil Moisture Gradients.
Miftahudin, Putri RE, Chikmawati T (2020) Vegetative morphophysiological responses of four rice cultivars to drought stress. Biodiversitas. 21(8):3727–3734. doi:10.13057/biodiv/d210840.
Mumtaz MZ, Saqib M, Abbas G, Akhtar J, Ul-Qamar Z (2020) Drought Stress Impairs Grain Yield and Quality of Rice Genotypes by Impaired Photosynthetic Attributes and K Nutrition. Rice Sci. 27(1):5–9. doi:10.1016/j.rsci.2019.12.001.
Panda DP, Ishra SSM, Ehera PKB (2021) Drought Tolerance in Rice : Focus on Recent Mechanisms and Approaches. 28 March 2020. doi:10.1016/j.rsci.2021.01.002.
Pandey V, Shukla A (2015) Acclimation and tolerance strategies of rice under drought stress. Rice Sci 22 (4): 147-161.
Patel DP, Das A, Munda GC, Ghosh PK, Sandhya J, Kumar M (2010) Evaluation of yield and physiological attributes of high-yielding rice varieties under aerobic and flood-irrigated management practices in mid-hills ecosystem. Agric Water Manag. 97(9):1269–1276. doi:10.1016/j.agwat.2010.02.018.
Rahmawati D, Dewi AK, Mufikasari VY, Wilujeng EDI, Adnan MR (2024) Agronomical Performances of Gajah Mungkur Mutant Rice Varieties Under Drought Stress. J Trop Life Sci. 14(1):65–76. doi:10.11594/jtls.14.01.08.
Rane J, Singh AK, Kumar M, Boraiah KM, Meena KK, Pradhan A, Vara Prasad P V (2021) The adaptation and tolerance of major cereals and legumes to important abiotic stresses. Int J Mol Sci. 22(23). doi:10.3390/ijms222312970.
Raumjit N, Siwadon S, Sukanya S, Thirayut W (2019) Comparison of root system and stomata in nine upland rice varieties. Int J Agric Technol. 15(5):769–778.
Redfern SK, Azzu NA, Binamira JS (2012) Rice in Southeast Asia: facing risks and vulnerabilities to respond to climate change. February:1–14. http://www.fao.org/3/i3084e/i3084e18.pdf.
Salsinha YCF, Indradewa D, Purwestri YA, Rachmawati D (2020) Selection of drought-tolerant local rice cultivars from east nusa tenggara, Indonesia during vegetative stage. Biodiversitas. 21(1):170–178. doi:10.13057/biodiv/d210122.
Sandar MM, Ruangsiri M, Chutteang C, Arunyanark A, Toojinda T, Siangliw JL (2022) Root Characterization of Myanmar Upland and Lowland Rice in Relation to Agronomic and Physiological Traits under Drought Stress Condition. Agronomy. 12(5). doi:10.3390/agronomy12051230.
Serraj R, McNally KL, Slamet LI, Kohli A, Haefele SM, Atlin G, Kumar A (2011) Drought resistance improvement in rice: an integrated genetic and resource management strategy. Plant Prod Sci, 14: 1–14
Sikuku PA, Netondo GW, Onyango JC, Musyimi DM (2010) Effects of water deficit on physiology and morphology of three varieties of nerica rainfed rice (Oryza sativa L .). ARPN J Agric Biol Sci. 5(1):23–28.
Sims DA and Gamon JA (2002) Relationships Between Leaf Pigment Content and Spectral Reflectance across a Wide Range of Species, Leaf Structures and Developmental Stages. Remote Sensing of Environment, 81, 337-354. http://dx.doi.org/10.1016/S0034-4257(02)00010-X
Suleiman OS, Habila GD, Mamadou F, Abolanle MB, Olatunbosun NA (2022) Grain yield and leaf gas exchange in upland NERICA rice under repeated cycles of water deficit at reproductive growth stage. Agric Water Manag. 264 May 2021:107507. doi:10.1016/j.agwat.2022.107507.
Suralta RR, Batungbakal MYT, Bello JCT, Caparas LM, Lagunilla VH, Lucas KMD, Patungan JU, Siping AJO, Cruz JA, Cabral MCJ (2018) An enhanced root system developmental responses to drought by inoculation of rhizobacteria (Streptomyces mutabilis) contributed to the improvement of growth in rice. Philipp J Sci. 147(1):113–122.
Suralta RR, Inukai Y (2014) Dry matter production in relation to root plastic development , oxygen transport , and water uptake of rice under transient soil moisture stresses Dry matter production in relation to root plastic development , oxygen transport , and water uptake of rice . July 2010. doi:10.1007/s11104-009-0275-8.
Vijayaraghavareddy P, Xinyou Y in, Truik PCS, Makarla U, Sreeman S (2020). Responses of lowland , upland and aerobic rice genotypes to water limitation during different Phases. Rice Sci. 27(4). doi:10.1016/j.rsci.2020.05.009.
Wang H. (2009) Fractal analysis on root systems of rice plants in response to drought stress. Environ Exp Bot. March 2009. doi:10.1016/j.envexpbot.2008.10.002.
Wei X, Cang B, Yu K (2022) Physiological Characterization of Drought Responses and Screening of Rice Varieties under Dry Cultivation. Agronomy 12 (11): 2849. doi:0.3390/agronomy12112849.
Wilujeng EDI, Ningtyas W, Nuraini Y (2015) Combined applications of biochar and legume residues to improve growth and yield of sweet potato in a dry land area of East Java. Journal of Degraded and Mining Lands Management 2 (4): 377–382. doi: 10.15243/jdmlm.2015. 024.377
Wu XH, Wang W, Yin CM, Hou HJ, Xie KJ, Xie XL (2017) Water consumption, grain yield, and water productivity in response to field water management in double rice systems in China. PLoS One. 12(12). doi:10.1371/journal.pone.0189280.
Xu Q, Ma X, Lv T, Bai M, Wang Z, Niu J (2020) Effects of water stress on fluorescence parameters and photosynthetic characteristics of drip irrigation in rice. Water (Switzerland). 12(1). doi:10.3390/w12010289.
Yang X, Wang B, Chen L, Li P, Cao C (2019) The different influences of drought stress at the flowering stage on rice physiological traits, grain yield, and quality. Sci Rep. 9(1):1–13. doi:10.1038/s41598-019-40161-0.
Yoshida S. (1981). Fundamentals of Rice Crop Science. Manila: The International Rice Reseach Institute.
Zhang H, Xue Y, Wang Z, Yang J, Zhang J (2009) Field Crops Research Morphological and physiological traits of roots and their relationships with shoot growth in ‘“ super ”’ rice. Field Crop Research. 113:31–40. doi:10.1016/j.fcr.2009.04.004.
Zhang L, Zhang Z, Tao F, Luo Y, Zhang J, Cao J (2022) Adapting to climate change precisely through cultivars renewal for rice production across China: When, where, and what cultivars will be required? Agric For Meteorol. 316 November 2021:108856. doi:10.1016/j.agrformet.2022.108856.
Zhang YJ, Xu JN, Cheng YD, Wang C, Liu GS, Yang JC (2020) The effects of water and nitrogen on the roots and yield of upland and paddy rice. J Integr Agric. 19(5):1363–1374. doi:10.1016/S2095-3119(19)62811-X.
Zheng HW, Lian HZ, Jun MA, Xu LI, Yan LI, Rong PZ (2010) Effects of Water Stress on Reactive Oxygen Species Generation and Protection System in Rice During Grain-Filling Stage. Agric Sci China. 9(5):633–641. doi:10.1016/S1671-2927(09)60138-3.