

Aust J Crop Sci. 19(04):423-428 (2025) | ISSN:1835-2707
https://doi.org/10.21475/ajcs.25.19.04.p299
First report of paraquat-resistance Conyza sumatrensis in Bolivia and efficacy of herbicides for its control
Lucas Vitorio*1, Jorge Torrez Arteaga2, José Saavedra-Avila3
1Crop Protection Development, Syngenta Bolivia, Santa Cruz, Bolivia
2Centro de investigación Agrícola Tropical, Santa Cruz, Bolivia
3Colegio de Postgraduados, Departament of Genetics, Mexico
*Corresponding author: Lucas Vitorio 
 ORCID ID:
https://orcid.org/0000-0002-2143-0339
ORCID ID:
https://orcid.org/0000-0002-2143-0339
Abstract: Conyza sumatrensis is one of the main weeds affecting extensive agricultural crops in the department of Santa Cruz, Bolivia, causing yield losses due to difficulties in control with herbicides used in conventional management. In 2020, isolated Conyza sp. plants were detected in the town of San Pedro, raising suspicions of resistance to the herbicide paraquat. Subsequently, this phenomenon was observed in other regions. The objectives of this study were to determine the resistance of C. sumatrensis to paraquat and to evaluate the efficacy of different herbicides for its management. To this end, two greenhouse trials were conducted in 2023. The first experiment consisted of dose-response bioassays on whole plants using paraquat, while the second trial tested the herbicides 2,4-D, dicamba, fluroxypyr, and paraquat at doses of 720, 240, and 100 g ae ha⁻¹, and 400 g ai ha⁻¹, respectively. The dose-response analysis revealed a high resistance index (RI) in the Cuatro Cañadas biotype (R), with an RI of 24.8 compared to the Yapacaní biotype (S). The herbicides 2,4-D and dicamba proved to be effective for managing this weed. This study presents the first report of C. sumatrensis resistance to paraquat in Bolivia and proposes alternative herbicides for its control. Integrated weed management strategies should be implemented to mitigate resistance cases and delay the development of resistance to currently effective herbicides.
Keywords: resistance, herbicide, paraquat, auxin herbicides, control, sumatran fleabane.
Introduction
Among the group of weeds that are currently difficult to control, Conyza is one of the most significant weeds worldwide, causing major losses in various crops and requiring high investments in control measures (Florentine et al., 2021). One of the main factors contributing to the increasing importance of weeds is herbicide resistance, which is defined as the natural and heritable ability of weeds to survive and reproduce after herbicide application (Christoffoleti et al., 1994).
Conyza sumatrensis (Syn.: Erigeron sumatrensis) is a South American species that has become widely distributed globally due to its high adaptability to different climatic conditions (Pruski and Sancho, 2006; Florentine et al., 2021). It is characterized by a high small seed production rate, with small seeds equipped with a structure known as pappus, which allows them to be dispersed over long distances by wind or water (Gianelli et al., 2017; Florentine et al., 2021). Other factors contributing to the increasing significance of this weed include its excellent adaptation to the predominant cropping system, no-tillage, as it germinates more effectively on the soil surface (Florentine et al., 2021; Mahajan et al., 2021), and the alarming rise in cases of resistance to different herbicides (Baccin et al., 2022; Heap, 2024).
Yield losses due to competition are significant. For example, a closely related species (Conyza bonariensis) can cause up to 25% yield losses in some soybean cultivars (Trezzi et al., 2013). According to Agostinetto et al. (2018), an increase of just one plant per square meter can lead to yield losses ranging from 1.4% to 25.9%, depending on the competitive ability of the cultivar. Additionally, these weeds serve as hosts for various crop pests, including pentatomid bugs and defoliating larvae (Dalazen et al., 2016). Other studies on the interference of C. sumatrensis in early- and late-maturing soybean cultivars, under different infestation densities, have shown that even a single plant per square meter can reduce soybean productivity by 9.35% to 13.72%, depending on the cultivar and growing season (Lorenzetti et al., 2025).
Paraquat is a broad-spectrum, non-selective herbicide that acts on Photosystem I in chloroplasts. It has low translocation and acts rapidly, causing desiccation of green plant tissue within a few hours in the presence of light. It has no residual activity, as it is strongly inactivated by soil, allowing for immediate sowing without the risk of phytotoxicity. This characteristic makes it widely applicable in many crops, including minimum tillage and no-tillage systems (Bromilow, 2003).
This herbicide is recommended for the control of Conyza in small plants, as its efficacy is drastically reduced in plants measuring 16 to 25 cm in height (Schneider et al., 2021). For plants exceeding the optimal size, sequential application can be used (Werth et al., 2010; Schneider et al., 2021; Albrecht et al., 2022b).
Synthetic auxin herbicides (Group 4, HRAC) mimic the effect of the plant hormone known as auxin. They are recommended for the control of broadleaf weeds in different cropping systems. The most widely used herbicides with this mechanism of action are 2,4-D and dicamba, whose usage has been influenced by the development and adoption of herbicide-resistant crop technologies (Todd et al., 2020).
The herbicides 2,4-D and dicamba have been extensively studied, showing positive results for Conyza control in both standalone applications and combinations with other herbicides, primarily glyphosate (Albrecht et al., 2022a; Cantu et al., 2021; Florentine et al., 2021; Schneider et al., 2021). However, when targeting larger plants, a sequential application or double-knock strategy is required (Schneider et al., 2021; Lorenzetti et al., 2024). The double-knock technique consists of an initial application of systemic herbicides, such as glyphosate combined with auxinic herbicides (primarily 2,4-D), followed by a second application within 5-7 days using a fast-acting contact herbicide, such as paraquat, diquat, glufosinate, or saflufenacil, among others (Werth et al., 2010; Cantu et al., 2021; Florentine et al., 2021; Albrecht et al., 2022a; Albrecht et al., 2022b; Lorenzetti et al., 2024).
To date, 20 cases of resistance have been reported worldwide for C. sumatrensis, 9 of which involve resistance to photosystem I (PSI) inhibitors. However, no cases of resistance for this species have been reported in Bolivia (Heap, 2024). The first observations of paraquat control failures in Bolivia were recorded in the locality of San Pedro, Santa Cruz department, in May 2020, after which the resistance spread to other regions. For this reason, the objective of this study was to evaluate the resistance of C. sumatrensis to paraquat and assess the efficacy of different post-emergent herbicides for its control.
Results and discussion
Paraquat dose-response
The results of the dose-response analysis showed that the Cuatro Cañadas biotype had a resistance index (RI) of 24.18 compared to the Yapacaní biotype, requiring 5289.56 g ai ha⁻¹ to reduce its dry matter weight by 50%, equivalent to 13.2 times the recommended commercial dose (400 g ai ha⁻¹). In contrast, the Yapacaní biotype required only 218.75 g ai ha⁻¹ to achieve the same 50% reduction in dry matter weight (Fig. 1; Table 1).
Other studies have found resistance index (RI) values for dry weight ranging from 3.6 to 34 (variable) for different resistant biotypes (Zobiole et al., 2019), 51.83 in Brazil (De Pinho et al., 2019), 3.92 in Paraguay (Albrecht et al., 2020), 35.1 in China (Guo et al., 2022), and <5.0 in
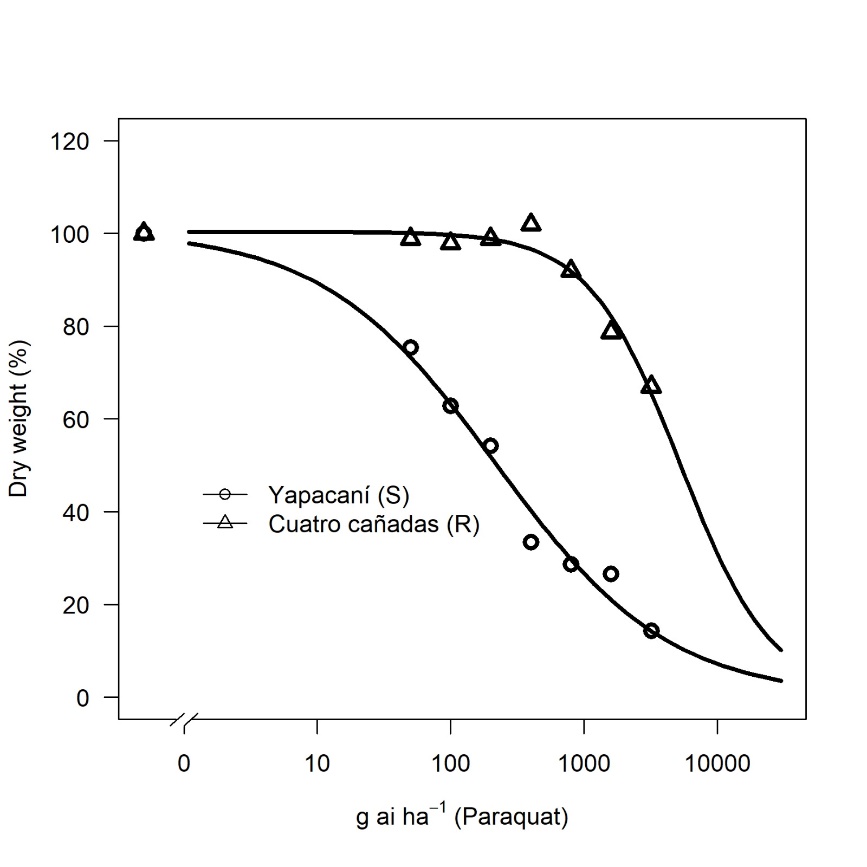
Figure 1. Dose-response curve of dry weight, 28 days after paraquat application.
Australia based on plant survival (Asaduzzaman et al., 2021). The RI for paraquat varies significantly across these studies, with the highest recorded value being 51.83 in Brazil (De Pinho et al., 2019). The resistance index (RI) indicates the degree of resistance, with higher values representing strong resistance, while lower values indicate weak resistance or susceptibility (RI = 1) (Asaduzzaman et al., 2021). RI values greater than 10, based on GR₅₀, are considered high resistance (Gazziero et al., 2008). We consider that the RI recorded in this study (24.18) is high, therefore we can deduce that increasing the herbicide dose as a strategy to improve control will not be effective. Therefore, alternative herbicides should be prioritized.
Globally, 9 cases of resistance to paraquat have been reported for C. sumatrensis in countries such as Taiwan (1980), Japan (1986), Malaysia (1990), Sri Lanka (1998), Brazil (1 case in 2016 and 2 cases in 2017), Paraguay (2017), and Australia (2018) (Heap, 2024). According to a resistance risk analysis for Conyza sp., paraquat resistance is classified as moderate risk when no management measures are implemented (Moss et al., 2019).
Two mechanisms of Conyza sp. resistance to paraquat have been described: the first is due to increased activity of the antioxidant system, and the second is due to reduced translocation and vacuolar sequestration (Baccin et al., 2022). However, the mechanism involved in paraquat resistance in Bolivia is still not fully understood.
The first reports of C. sumatrensis resistance to paraquat in Bolivia’s neighboring countries occurred in 2017 in Brazil and Paraguay (Heap, 2024), and this issue has now extended into Bolivia. In Bolivia, the first suspicions of paraquat resistance were observed in the locality of San Pedro, Santa Cruz department (May 2020), in experimental areas where herbicide trials were conducted on fallow land (Fig. 2A). Later, resistance was detected at the commercial level in different regions, both in fallow systems and within soybean crops following
Table 1. Three-parameter logistic model of the dry weight percentage variable in C. sumatrensis biotypes.
| Biotypes | b | d | GR50 | RI |
|---|---|---|---|---|
| Yapacaní (S) | 0.67 (±0.12)* | 100.65 (±1.19) | 218.75 (±53.55) | |
| Cuatro Cañadas (R) | 1.26 (±0.62) | 100.37 (±5.34) | 5289.56 (±1474.43) | 24.18 |
GR50: 50% growth reduction; RI: Resistance Index; *Standard Error.
Table 2. Percentage of control of paraquat-resistant C. sumatrensis with different herbicides.
| Treatments | 7 DAA | 14 DAA | 21 DAA | 28 DAA | |||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | Control | 0 | a | 0 | a | 0 | a | 0 | a |
| 2 | 2.4-D 720* | 64 | b | 85 | d | 96 | c | 99 | c |
| 3 | Dicamba 240* | 66 | b | 85 | d | 90 | c | 93 | c |
| 4 | Fluroxypyr 100* | 48 | b | 63 | c | 57 | b | 48 | b |
| 5 | Paraquat 400** | 9 | a | 13 | b | 8 | a | 3 | a |
| CV | 29.48 | 11.71 | 10.78 | 8.55 | |||||
| p-value | <0.0001 | <0.0001 | <0.0001 | <0.0001 | |||||
Means followed by the same letter in the column do not differ statistically from each other in Tukey’s test (p < 0.05). DAA: Days after application. CV: Coefficient of variation. *Herbicide in g ae ha-1. ** herbicide in g ai ha-1.
desiccation for harvest using paraquat. This indicates that the problem is spreading to different agricultural regions with extensive crop production (Fig. 2B). The expansion of resistant populations is favored by the characteristics of the seed, which has structures such as the pappus that facilitate transport mainly by wind (Gianelli et al., 2017; Florentine et al., 2021).
Early detection and eradication of initial escape foci after herbicide application are important, as their impact is lower at this stage. Once the infestation becomes widespread, eradication is unlikely (Simard and Laforest, 2024).
The detection of paraquat-resistant C. sumatrensis requires a rethinking of current weed management strategies, and multiple integrated approaches should be adopted, including: the use of different herbicides with proven efficacy, the efficient application of the double-knock technique, and the application of pre-emergent herbicides (Cantu et al., 2021). Additionally, soil cover management and crop rotation are also recommended for controlling Conyza (Bajwa et al., 2016). These strategies have been widely implemented in other parts of the world. However, locally, although reactive management measures are applied in the field, published information remains very scarce, presumably even non-existent. This reflects the urgent need for more local research on this increasingly serious issue, as it is becoming more challenging to control resistant weeds, leading to higher production costs and yield losses.
Efficacy of herbicides on resistant Conyza sumatrensis
The highest levels of control (%) were achieved with the herbicides 2,4-D (720 g ae ha⁻¹) and dicamba (240 g ae ha⁻¹), reaching their peak efficacy 28 days after application (DAA). In contrast, the herbicide fluroxypyr (100 g ai ha⁻¹) showed lower control levels, and after 14 DAA, its efficacy declined as the plants recovered from the herbicide. Similarly, paraquat efficacy on this resistant biotype was the lowest, achieving only 13% control at its best performance (Fig. 4; Table 2).
The herbicides 2,4-D and dicamba are effective alternatives for controlling C. sumatrensis, even in populations resistant to paraquat. The response of this biotype to paraquat was very low, further corroborating
the strong resistance observed in the initial dose-response trial.
The application of auxinic herbicides is recommended on young plants for optimal results (Florentine et al., 2021). Some herbicides in this group, such as 2,4-D, dicamba, and triclopyr, have proven highly effective in controlling this weed (Cantu et al., 2021; Florentine et al., 2021).
Guo et al. (2022) confirmed that fluroxypyr is not an optimal alternative for controlling C. sumatrensis, as it is effective only at very early growth stages of only 4 to 5 leaves, while larger plants exhibit high tolerance. According to Albrecht et al. (2022a), glyphosate combined with auxinic herbicides (such as 2,4-D, dicamba, and triclopyr) demonstrated greater efficacy than the combination of fluroxypyr + glyphosate + clethodim. In another study, glyphosate + dicamba was more effective than glyphosate + fluroxypyr when applied to plants up to the 14-leaf stage; however, a sequential application was required for optimal results (Cassol et al., 2024). The findings of these authors align with those observed in this study, where fluroxypyr was less effective than other auxinic herbicides, such as 2,4-D and dicamba, when applied to plants at the 8- to 9-leaf stage.
Although fluroxypyr, 2,4-D, and dicamba are all auxinic herbicides, they exhibit variable efficacy across different weed families and species. Possible causes may include physiological differences (e.g., cuticle, trichome density), variations in metabolism, and sensitivity at the target site (Todd et al., 2020)
In Brazil, resistance of C. sumatrensis to 2,4-D has been detected, an unusual phenomenon known as rapid necrosis, in which herbicide effects appear as partial tissue burn just 15 minutes after application (De Queiroz et al., 2019). However, this type of resistance to 2,4-D does not appear to affect other auxinic herbicides, such as dicamba (De Queiroz et al., 2019; Lorenzetti et al., 2024). The existence of this type of resistance to 2,4-D in Brazil should raise concerns in Bolivia, given the proximity and shared national borders between the two countries. If this form of 2,4-D resistance were to spread to Bolivia, the results of this study indicate that alternative auxinic herbicides with proven efficacy are available.
Research lines on resistant weeds require a holistic approach such as: analysis of control failures and their

Figure 2. (A) Surviving isolated Conyza seedling after paraquat application, San Pedro location, May 2020; (B) Surviving Conyza plants after paraquat application during pre-harvest soybean desiccation, Cuatro Cañadas location, March 2023.

Figure 3. Control of C. sumatrensis in the dose-response test to paraquat. Yapacaní, susceptible biotype (S); Cuatro Cañadas, resistant biotype (R).

Figure 4. Efficacy of different herbicides on the C. sumatrensis biotype resistant to paraquat herbicide at 7, 14, 21, and 28 days after application (DAA).
geographical distribution, confirmation of resistance, analysis of the resistance mechanism, testing of alternative control methods and evaluation of the sustainability of management strategies (Liu et al., 2020). In the results of the work presented here, we recorded field control failures, determined resistance and presented efficient herbicides to manage paraquat resistance control.
Although the herbicides proposed for managing C. sumatrensis have shown efficiency, it is crucial to implement additional integrated management strategies to mitigate and delay resistance in Bolivia’s agricultural regions.
Materials and Methods
Paraquat dose-response bioassay
Conyza sp. Conyza sp. seeds were collected from soybean fields between July 2022 and March 2023. In May 2023, 10 biotypes of Conyza sp. were screened against different herbicides. The F2 generation of two biotypes, selected from the localities of Yapacaní (-17.406517; -63.917777) and Cuatro Cañadas (-17.094582; -62.6112414), which exhibited differential sensitivity, were chosen for dose-response tests. Species-level identification of the selected biotypes was conducted on reproductive-stage plants, following the identification keys developed by Pruski and Sancho (2006) and Maslo and Šarić (2021).
The experimental design used was completely randomized blocks with 5 replicates, where each pot was considered an experimental unit. Treatments consisted of increasing doses of paraquat: 0, 50, 100, 200, 400, 800, 1600, and 3200 g ai ha⁻¹. The recommended field dose (1X) was 400 g ai ha⁻¹, and the commercial formulation used in the experiment was Gramoxone (paraquat 200 g L⁻¹). Mineral oil (Nimbus) was added at 0.5% v/v to all treatments except the untreated control.
The application was carried out on October 22, 2023, on seedlings at the 6–8 leaf stage, using CO₂-driven application equipment with XR-11002 flat-fan nozzles (TeeJet), calibrated to apply a volume of 150 L ha⁻¹. After application, the plants were maintained under constant irrigation in a greenhouse.
Methodological aspects, such as seed collection, plant maintenance in the greenhouse, and application, were performed following the recommendations of Burgos (2015).
The evaluation of dry matter weight was conducted 28 days after application, during which the aerial part of each plant was cut, then dried before measuring the weight. Subsequently, the dry weight data were converted into percentages relative to their untreated controls. A three-parameter logistic model equation [1] was fitted, with adjustment for standard errors, where GR50 denotes the necessary paraquat dose to inhibit dry weight by 50% (Ritz et al., 2015).
\[y = \frac{d}{1 + exp(b\left( \log(x) - \log(e) \right))}\ \ \ \ \ \ \ \ \ \ \ \ \ \ \ \ \ \ \lbrack 1\rbrack\ \ \ \ \ \ \ \]
Efficacy of resistant Conyza sumatrensis herbicides
This trial was conducted in a greenhouse on a paraquat-resistant biotype of C. sumatrensis, which was confirmed in the first dose-response bioassay.
A completely randomized block design with 6 replicates was used. The treatments tested were: 2,4-D (720 g ae ha⁻¹, Campeon), dicamba (240 g ae ha⁻¹, Rainvel), fluroxypyr (100 g ae ha⁻¹, Starane Xtra), paraquat (400 g ai ha⁻¹, Gramoxone), and a control treatment without herbicide. Mineral oil (Nimbus) was added at a dose of 0.5% v/v to the paraquat treatment.
The application was made on seedlings with 8–9 leaves, using CO₂-driven application equipment equipped with XR-11002 flat-fan nozzles (TeeJet) and an application volume of 150 L ha⁻¹.
Herbicide efficacy was evaluated at 7, 14, 21, and 28 DAA, through visual estimation of the percentage of plant control compared to the untreated control, where 0% indicated no damage and 100% indicated complete plant death
The % control data were subjected to analysis of variance, and means were compared using Tukey's test (p < 0.05) with the statistical program Infostat (Di Rienzo et al., 2020).
Conclusion
The results confirm the finding of C. sumatrensis resistance in the Cuatro Cañadas biotype to the herbicide paraquat. This is the first report of resistance in this important weed to a widely used herbicide in Bolivian agriculture. The herbicides 2,4-D and dicamba are efficient control alternatives for the biotype with confirmed resistance to paraquat. This discovery raises an alert and highlights the urgent need to incorporate integrated weed management practices. Further studies are required to understand the mechanism of resistance of this weed to paraquat, as well as monitoring a broader range of herbicides to assess their efficacy and resistance status.
Acknowledgments
To Bladimir Torrez for his collaboration in seed collection and in the application of the screening work.
References
Agostinetto D, Silva DRO, Vargas L (2017) Soybean yield loss and economic thresholds due to glyphosate resistant hairy fleabane interference. Arquivos do Instituto Biológico. 84:e0022017.
Albrecht AJP, Thomazini G, Albrecht LP, Pires A, Lorenzetti JB, Danilussi MTY, Silva AFM, Adegas FS (2020) Conyza sumatrensis resistant to paraquat, glyphosate and chlorimuron: confirmation and monitoring the first case of multiple resistance in Paraguay. Agriculture. 10:582.
Albrecht LP, Albrecht AJP, Silva AFM, Ramos RA, Da Costa KYR, De Araujo GV, Mundt TT, Colombari C (2022) Sequential application of herbicide options for controlling Conyza sumatrensis in soybean pre-sowing. Rev Fac de Ciencias Agrarias UNCuyo. 54:83-93.
Albrecht LP, Heimerdinger N, Albrecht AJP, Silva AFM, Piccin ES, Da Silva LM, Larini WFM (2022a). Chemical Control of Fleabane Resistant to 2,4-D. Outlooks Pest Manag. 33:239-243.
Asaduzzaman M, Koetz E, Wu H, Shephard A (2021) Paraquat resistance and hormetic response observed in Conyza sumatrensis (Retz.) E. Walker (tall fleabane) in Australian cotton cropping systems. Phytoparasitica. 50:269-279.
Baccin LC, Albrecht AJP, Albrecht LP, Silva AFM (2022) Mechanisms of multiple resistance to herbicides in Conyza sp. complex. J Plant Protect Res. 62:113-121.
Bajwa AA, Sadia S, Ali HH, Jabran K, Peerzada AM, Chauhan BS (2016) Biology and management of two important Conyza weeds: a global review. Enviro Sci Pollut Res. 23:24694-24710.
Bromilow RH (2003) Paraquat and sustainable agriculture. Pest Manag Sci. 60:340-349.
Burgos NR (2015) Whole-Plant and seed bioassays for resistance confirmation. Weed Sci. 63:152-165.
Cantu RM, Albrecht LP, Albrecht AJ, Silva AF, Danilussi MT, Lorenzetti JB (2021) Herbicide alternative for Conyza sumatrensis control in pre-planting in no-till soybeans. Weed Sci. 39:e2021000025.
Cassol M, Albrecht A, Albrecht L, Silva A, Larini W, Galvão F (2024) Mixtures of herbicides for the control of multiple resistant Conyza sumatrensis in soybean pre-sowing burndown. Revista Brasileira de Ciências Agrárias - Braz J Agric Sci. 19:1-8.
Christoffoleti PJ, Filho RV, Da Silva CB (1994) Resistência de plantas daninhas aos herbicidas. Planta Daninha. 12:13-20.
Dalazen G, Curioletti LE, Cagliari D, Stacke RF, Guedes JVC (2016) Hairy fleabane as a source of major insect pests of soybean. Planta Daninha. 34:403-409.
De Pinho CF, Leal JFL, Souza, ADS, De Oliveira, GFPB, de Oliveira C, Langaro AC, Machado AFL, Christoffoleti PJ, Zobiole LHS (2019) First evidence of multiple resistance of Sumatran Fleabane (Conyza sumatrensis (Retz.) E. Walker) to five-mode-of-action herbicides. Aust J Crop Sci. 13:1688-1697.
De Queiroz ARS, Delatorre CA, Lucio FR, Rossi CVS, Zobiole LHS, Merotto A (2019) Rapid necrosis: a novel plant resistance mechanism to 2,4-D. Weed Sci. 68:6-18.
Di Rienzo JA, Casanoves F, Balzarini MG, Gonzalez L, Tablada M, Robledo CW (2020) InfoStat versión 2020. Centro de Transferencia InfoStat, FCA, Universidad Nacional de Córdoba, Argentina.
Florentine S, Humphries T, Chauhan BS (2021) Erigeron bonariensis, Erigeron canadensis, and Erigeron sumatrensis. In: Chauhan B (ed) Biology and Management of Problematic Crop Weed Species. Elsevier, London.
Gazziero DLP, Christoffoleti PJ, Vargas L, Kruse ND, Galli AJB, Trezzi MM (2008) Critérios para relatos oficiais estatísticos de biótipos de plantas daninhas resistentes a herbicidas. In Gazziero DLP, Galli AJB, Karam, D (eds), 1st edn. Sete Lagoas, Brazil.
Gianelli V, Bedmar F, Ulzurrun PDD, Panaggio H (2017) Dinámica de emergencia y competencia intraespecífica en Conyza sumatrensis. Agrociencia. 21:69-77.
Guo WL, Yu CJ, Zhang C, Zhang TJ, Tian XS (2022) Multiple resistance detection to glyphosate and other herbicides in Conyza sumatrensis and the evaluation of chemical control herbicides. Chin J of Pestic Sci. 24:789-797.
Heap I (2024) The International Herbicide-Resistant Weed Database. Available www.weedscience.org (accessed March 25, 2024).
Liu C, Jackson LV, Hutchings S, Tuesca D, Moreno R, Mcindoe E, Kaundun SS (2020) A holistic approach in herbicide resistance research and management: from resistance detection to sustainable weed control. Sci Rep. 10:1.
Lorenzetti JB, Albrecht AJP, Albrecht LP, Danilussi MTY, Barroso AAM, Bauer FE, Silva AFM, Marchi CS (2025) Interference of Conyza sumatrensis on grain yield of soybean cultivars. Revista Facultad Nacional de Agronomía Medellín. 78:10967-10975.
Lorenzetti JB, Danilussi MTY, Albrecht AJP, Barroso AAM, Albrecht LP, Silva AFM, Santos GRD, Caneppele GAM (2024) Identification, mapping, and chemical control of fleabane resistant to glyphosate, chlorimuron, paraquat, and 2,4-D. Weed Technol. 38:e27.
Mahajan G, Prasad A, Chauhan BS (2021) Seed germination ecology of Sumatran fleabane (Conyza sumatrensis) in relations to various environmental parameters. Weed Sci. 69:687-694.
Maslo S, Šarić Š (2020) Erigeron sumatrensis Retz. (Compositae), a recently recognized invasive alien species in Bosnia and Herzegovina. Glasnik Hrvatskog botaničkog društva. 8:88-93.
Moss S, Ulber L, Hoed ID (2019) A herbicide resistance risk matrix. Crop Prot. 115:13-19.
Pruski JF, Sancho G (2006) Conyza sumatrensis var. leiotheca (Compositae: Astereae), a new combination for a common neotropical weed. Novon. 16:96-101.
Ritz C, Baty F, Streibig JC, Gerhard D (2015) Dose-response analysis using R. PloS one. 10:e0146021.
Schneider NT, Camera JN, Koefender J, Rizzardi MA, Bianchi MA, Rockenbach AP (2021). Herbicide performance in the control of Conyza spp. where three plant heights. Biosci J. 37:e37091.
Simard M, Laforest M (2024). Early detection and management of herbicide resistant weeds. Can J Plant Sci, 104:533-539.
Todd OE, Figueiredo MR, Morran S, Soni N, Preston C, Kubeš MF, Napier R, Gaines TA (2020) Synthetic auxin herbicides: finding the lock and key to weed resistance. Plant Sci. 300:110631.
Trezzi MM, Balbinot JrAA, Benin G, Debastiani F, Patel F, Miotto JrE (2013) Competitive ability of soybean cultivars with horseweed (Conyza bonariensis). Planta Daninha. 31:543-550.
Werth J, Walker S, Boucher L, Robinson G (2010) Applying the double knock technique to control Conyza bonariensis. Weed Biol Manage. 10:1-8.
Zobiole LHS, Pereira VGC, Albrecht AJP, Rubin RS, Adegas FS, Albrecht LP (2019) Paraquat resistance of Sumatran fleabane (Conyza sumatrensis). Planta Daninha. 37:e019183264.