

Aust J Crop Sci. 19(04):449-457 (2025) | ISSN:1835-2707
https://doi.org/10.21475/ajcs.25.19.04.p227
Carbon flux and net primary production in mineral soil-based tropical agricultural land: a study case corn and peanut crops
Hermanu Widjaja1,3, Suwardi1,3*, Dyah Tjahyandari Suryaningtyas1,3, Putri Oktariani1,3, Vecky Dwi, Kuswandora2
1Department of Soil Science and Land Resources, Faculty of Agriculture, IPB University, Indonesia
2BiOWiSH Technologies International, Inc 2717 Erie Ave, Cincinnati, OH 45208, United States of America
3Center for Mine Reclamation Studies, International Research Institute for Environment and Climate Change, IPB University, Indonesia
*Corresponding author: Suwardi 
Abstract: Agriculture is a major contributor to greenhouse gas emissions, and crop management practices can influence carbon dioxide (CO2) and methane (CH4) emissions. This study aimed to evaluated the crop yield and carbon fluxes of corn and peanut crops. The research was conducted from November to June during the dry season in Ranca Bungur District, Bogor, West Java, Indonesia, where we monitored soil CO2 and CH4 fluxes alongside Net Primary Production (NPP) in corn and peanut fields. Carbon flux was measured using the closed chamber method, and NPP was calculated by multiplying the total plant biomass at harvest by its carbon content. The result showed that the corn field had higher CO2 emissions (4.35 ± 1.09 g C-CO2 m-2 d-1) compared to the peanut field (2.59 ± 0.71 g C-CO2 m-2 d-1), while CH4 emissions were low in both fields but slightly higher in the peanut field (0.26 ± 0.69 mg C-CH4 m-2 d-1) than in the corn field (0.08 ± 0.46 mg C-CH4 m-2 d-1). Furthermore, the study found that corn had a higher NPP than peanuts, resulting in a positive correlation between carbon emission and NPP in both fields. The study suggests that increasing NPP could reduce carbon emissions and fix more carbon into the system.
Keywords: Arachis hypogaea, CO2 flux, CH4 uptake, Zea mays.
Introductions
In recent years, climate change has been a concern for many researchers. They have predicted a significant increase in the temperature of the atmosphere and oceans due to the emission of greenhouse gases (GHG) such as carbon dioxide (CO2), methane (CH4), nitrous oxide (N2O), tropospheric ozone (O3) and chlorofluorocarbons (CFCs) (IPCC, 2007). Soil, recognized as the most terrestrial carbon sink, stores more carbon than the combined biomass and atmosphere of the planet. The top meter of global soils contains approximately 2200 petagrams (Pg) of carbon, making it the most substantial terrestrial carbon reservoir, with around 1500 Pg specifically as soil organic carbon (Sharififar et al., 2023). The degradation of soil, marked by the loss of organic carbon, is a primary indicator of land degradation (Nijbroek et al., 2018).
Land degradation is a complex term in geographical literature and environmental studies due to its association with similar phenomena such as deforestation, desertification, and soil erosion. Land degradation refers to the impairment of land quality and its associated elements caused by natural or anthropogenic factors. This process affects the value of the biophysical environment, leading to changes or disturbances deemed detrimental to the ecosystem and its inhabitant (Kaiser, 2020). Land degradation can be caused by an increase in greenhouse gases emission include the reduction of ecosystem services such as carbon sequestration, climate change, and decrease in biodiversity or water storage. Climate change represents one of the most significant threats humanities has ever faced. It is a crucial factor in agricultural production systems, directly and indirectly affecting cultivation. Climate profoundly impacts biogeochemical cycles in soil, as all three types of soil processes—physical, chemical, and biological—are governed by the prevailing climatic conditions of a particular location (Ghosh and Mandal, 2023). Additionally, nutrient cycling is an indicator for the reduction of ecosystem services related to soil and change in land use for agricultural land (Ryusuke et al., 2015).
According to the Intergovernmental Panel on Climate Change (IPCC), land degradation is a driver of climate change through emission of greenhouse gases (GHGs) and reduced rates of carbon uptake (IPCC, 2022). On agricultural land, the choice of cropping system and the implementation of crop management practices influence CO2, CH4, and N2O emissions (IPCC, 1997). According to (Raich and Schlesinger, 1992) CO2 emission resulting from soil respiration is 10 to 15 times higher than fossil fuels emmisions. Globally, agricultural production (crops and livestock) is responsible for the majority of methane emission (from cattle pastures, rice farms, and wetlands) and nitrous oxide (due to the intensive application of fertilizer). Therefore, the potential for technical mitigation in the agricultural sector is high, and 74% of it is in developing countries (FAO, 2011)
The contribution of agricultural soils to CO2, N2O and CH4 emissions depends on the biophysical processes, and the incorporation/decomposition of organic residues in the soil (Badewa et al., 2022). Aerobic soil conditions produce CO2, while anaerobic conditions produce CH4, and mineral-N nitrification and denitrification processes result in into N2O emission (Muñoz et al., 2010). Carbon is essential as energy in denitrification. The low availability of carbon result in low denitrification and it is responsible for the abundance of NO3-N concentration in the soil (Nugroho et al., 2015).
There is growing concern about global warming and rising concentrations of greenhouse gases in the soil. In terrestrial ecosystems, soil plays a crucial role in the transforming of carbon through various processes such as climate, regulate the cycles and movements of both organic and inorganic forms of these elements (Medhi et al. 2021). Soil tillage significantly impacts CO2 flux during the tillage process (Reicosky, 1997; Jia et al., 2021). Soil CO2, formed during microbial breakdown, is stored in soil pores and can be released into the atmosphere through diffusion or diffusion combined with mass flow. Methanogens play a crucial role in CH4 production, while methanotrophs consume it. These microorganisms thrive in soil or ocean sediments (Mehra et al., 2018). The activity of microorganisms and plant root systems in the soil can affect CO2 emissions and water content, and also high temperatures can increase CO2 emitted from the soil (Kuswandora, 2012a). Additionally, soil temperature, air temperature, humidity, and litter volume contribute to the CO2 flux, providing substrate food for soil microbes (Hendri et al. 2015).
Carbon naturally enters any terrestrial ecosystem by photosynthesis in plants. This process involves extracting carbon dioxide from the air, separating the carbon from the oxygen atoms, returning oxygen into the atmosphere, and using the arbon to make biomass in the form of roots, stems, foliage, and other plant parts. This process is commonly referred to as “carbon sequestration”, indicating that it is a natural process that removes carbon dioxide from the atmosphere and stores it in the soil (Chapin et al. 2011; Matthews, 2023). Plants take in CO2 from the atmosphere as they grow. Carbon is returned to the atmosphere mainly as CO2 from the metabolic respiration of plants, animals, and microbes, making up an important segment of the carbon cycle. It is also important to understand that net CO2 emissions result from the amount of atmospheric carbon, fixed through photosynthesis and stored in the soil as organic matter, and the amount of soil carbon oxidized to CO2 during a given period. It is necessary to consider that the primary source of net CO2 emissions in the atmosphere is associated with agricultural practices. The life cycle emissions of agricultural inputs were found to contribute to net GHG emissions through combined soil emissions of N2O and CH4, and CO2 emissions (Gao et al., 2018).
Carbon balance can be defined as the difference between the carbon assimilated by plants through of photosynthesis and the ecosystem level CO2 released by autotrophs and heterotrophs (Manzoni et al., 2018). On a broader scale, the carbon balance of ecosystems includes both the carbon stock in vegetation and soil and the carbon absorbed by vegetation (Chuai et al., 2019). If the amount of carbon absorbed by plants through photosynthesis is greater than the amount of carbon released by respiration, the ecosystem acts as a carbon sink. In this case, the ecosystem accumulates carbon in the atmosphere and the soil. Conversely, if the amount of carbon released by respiration exceeds the amount of carbon absorbed by plants, the ecosystem becomes a carbon source and releases carbon into the atmosphere (National Geographic Society, 2023). Towards determining carbon sink and source, FAO has developed several methods of evaluating carbon balance. Carbon balance assessment could help in building new strategies to adapt and prevent climate change consequences especially in the developing agricultural sector (Bernoux et al., 2010).
Agricultural lands could be globally significant sinks or sources of atmospheric CO2. However, the carbon balance of these areas still needs to be better quantified because most research has focused on CO2 emission from the soil only, without incorporating the carbon uptake by vegetation and additional carbon flows such as decomposition and leaching (Heimsch et al., 2021). Additionally, forest soils are crucial role as significant terrestrial sinks for atmospheric CH4. However, the intricate microbial production and oxidation processes, CH4 movement in forest ecosystems, and their connections to environmental controls still needs to be better understood (Feng et al., 2020). The overall amount of organic carbon added into the soil through living roots, as well as net rhizodeposition is defined as the part of the carbon that remained in the soil after microbial utilization and partial decomposition to CO2. So that carbon allocation from plant is complicated to measure (Pausch and Kuzyakov, 2018). Therefore, soil respiration is a crucial process that needs careful attention for various reasons, including understanding how the land's living environment interacts with the atmosphere and creating budgets for carbon within ecosystems.
The objective of this research was to assess the amount of carbon flux (CO2 and CH4) and Net Primary Production (NPP) of different uses of tropical agricultural in mineral soils.
Results and Discussion
Soil CO2 and CH4 flux
Total carbon loss in this study, which was made up of CO2 and CH4 fluxes, was observed to be higher in the corn field. The mean CO2 flux in the corn field reached 4.35 ± 1.09 g C-CO2 m-2 d-1, with a recorded range of 1.86 to 8.19 g C-CO2 m-2 d-1; while in the peanut field it amounted to 2.59 ± 0.71 g C-CO2 m-2 d-1 with a range of 1.44 to 4.01 g C-CO2 m-2 d-1 (Table 1).
These values are in general agreement with those that had been previously reported by other researchers. For instance, (Fan et al., 2019) observed that the average soil CO2 fluxes in maize growing seasons were 4.06, 4.01, 3.61 and 3.81 g m−2 d−1. The field experiment was carried out in 2014 and 2015 at the experimental station of Gansu Agricultural University, China. (Rumbang et al. 2009) also found that in a corn field in a West Kalimantan peatland that was measured in 2006 and 2007, the mean CO2 emissions were 0.31 and 0.39 g CO2 m-2 h-1, respectively. Similarly, in a Central Kalimantan peatland that was also planted to corn and monitored in 2005, 2006 and 2007, the mean CO2 emissions in a corn field after 1-5 years of cultivation were 0.24, 0.52, and 0.29 g CO2 m-2 h-1, respectively. In another field observed after 6-10 years of cultivation with corn, the corresponding values were 0.43, 0.81, 0.77 g CO2 m-2 h-1, respectively.
Methane fluxes ranged from –1.10 mg C-CH4 m-2 d-1 (uptake) to 3.11 mg C-CH4 m-2 d-1 (production), indicating that both methanotrophs (CH4 oxidizing bacteria) and methanogens (CH4 producing bacteria) were present in the soil microbial community at this site. Overall, oxidative processes dominated over production, with a mean flux rate in the corn field of 0.08 ± 0.46 mg C-CH4 m-2d-1, and 0.26 ± 0.69 mg C-CH4 m-2 d-1in the peanut field.
The results showed that the total CO2 flux in the corn field during the growing season (lasting 77 days each) was 3.308 ton C- CO2 ha-1, and in the peanut field (with a growing period of 75 days per season), it amounted to 2.028 ton C- CO2 ha-1. The total CH4 flux from the corn and peanut fields came out very low, at only 0.065 kg C-CH4 ha-1 and 0.186 kg C-CH4 ha-1, respectively (Figure 2). In an earlier study, (Rochette et al. 1999) found that for corn in eastern Canada, root respiration was zero over the first 30 days from planting, but during the next 30 days of plant growth, the contribution of root respiration increased linearly to a maximum of 45% where it remained constant until plant senescence. Total soil CO2 flux during the 160-day period from planting to harvest was 5.5 mg CO2–C ha-1, with root respiration accounting for 28.7% of this total seasonal soil respiration. Meanwhile, maize was growth slowly and root distributed shallow in early time, with the rapid vegetative growth and reproductive growth of maize, soil water storage of treatments gradually decreased. The soil CO2 fluxes showed a seasonal variation and fluctuated with the soil and the atmospheric temperature for upland in China (Fan et al., 2019).
(Li et al., 2016) reported that CH4 fluxes in peanut field among the sampling sites showed a higher CH4 fluxes, whereas the fluxes in corn field indicated the lower CH4 flux. The CO2 and CH4 flux in corn field and peanut field may be low due to organic matter, pH, water temperature, and photosynthetic active radiation. In corn field higher than peanut mainly due to fertilizer requirements. Peanut requires less fertilizer and water than corn (Feng et al., 2021), this can result in lower microbial activity in the soil, which can lead to lower CO2 fluxes.
CH4 has a global warming potential 28 times greater than that of CO2 on a 100-year timescale and directly contributes to approximately 20% of recent climate
Table 1. Mean (±SE), minimal and maximal of methane and carbon dioxide emissions at the corn and peanut fields.
| Carbon dioxide | Methane | |
|---|---|---|
| g C m-2 d-1 | mg C m-2 d-1 | |
| Corn | ||
| Mean | 4.35 ± 1.09 | 0.08 ± 0.46 |
| Minimum | 1.86 | 1.10 |
| Maximum | 8.19 | 1.23 |
| N | 36 | 39 |
| Peanut | ||
| Mean | 2.59 ± 0.71 | 0.26 ± 0.69 |
| Minimum | 1.44 | 1.13 |
| Maximum | 4.01 | 3.11 |
| N | 27 | 27 |
warming, despite its concentration being two orders of magnitude lower than that of CO2. This phenomenon is particularly evident in the context of methane emissions from mangrove wetland soils (Zheng et al., 2018). The amount of CH4 emission in the corn field was 0.065 kg C-CH4 ha-1 period-1 which is equal to 0.001 ton C-CO2 ha-1 period-1. Correspondingly, in the peanut field it measured 0.186 kg C-CH4 ha-1 period-1 which is equal to 0.004 ton C-CO2 ha-1 period-1. This number is very small. Hence, total soil carbon (CO2 and CH4) emission from the corn and peanut fields during the 77-day and 75-day period from planting to harvest amounted to 3.310 and 2.033 ton C ha-1, respectively. Further, logically, outside the cropping period, when the land is idle or bare (without crop), emission can be expected to be much lower. Soil CO2 flux consists of autotrophic respiration of plant roots and heterotrophic respiration of soil organisms. It also includes respiration from the litter layer above the mineral soil. The amount of soil CO2 flux is commonly referred to as soil respiration (Jiang et al., 2020). In this research though, soil respiration (CO2 flux) refers to the sum of heterotrophic respiration and autotrophic respiration in the soil. In according to (Warner et al., 2019) soil respiration refers to the exchange of CO2 between the soil and the atmosphere, which is generated by plant roots and microorganisms. The respiration flux has a significant impact on the carbon balance.
The peak CO2 flux was up to 8.19 g C-CO2 m-2 d-1 in-row chamber in the corn field. Significantly lower fluxes were recorded at the peanut field (1.44 to 4.01 g C-CO2 m-2 d-1). Fig 2. shows that the corn field on-row CO2 flux is higher than that inter-row. This indicates that CO2 flux was strongly influenced by the respiration of plant roots and the high activity levels of microorganisms around the roots. The farther away from the roots, the lower the CO2 flux. The lower CO2 emissions in the peanut field may be explained by the smaller contribution of plant root autotrophic respiration. The contribution of root respiration to total soil respiration is dependent on vegetation type, growing patterns, season, soil, climate, and management conditions. Management practices by soil plowing replaced sub-soil with top soil and made the dead root abundant on the top soil. It stimulated decomposition, hence, increased CO2 flux (Nugroho et al. 2018).
Methanogenic bacteria are a type of anaerobic bacteria that generate methane gas during their metabolic
Fig 1. CO2 measurement using a closed-chamber method. The CO₂ flux was measured using a closed-chamber method with a chamber base (3 cm depth) to prevent gas leakage. A stainless steel chamber (20 cm diameter, 26 cm height) was placed on the base. The chamber cover includes three ports: a syringe port for gas sampling, a tedlar bag port for airtight gas collection, and a pressure bag port to balance internal and atmospheric pressure.
processes (Shukla et al. 2021; Whitman et al. 2006). Methane formation occurs via anaerobic fermentation because of the nature of these bacteria. In livestock waste, the quantity of methane produced is directly proportional to the total amount of anaerobic bacteria present (Marlina et al., 2018). (Suprihati et al., 2006) suggested that CH4 gas is produced by biological activity of the microbial agents (methanogen bacteria) through decomposition or decay of organic matter that occurs in paddy fields and fermentation in ruminant animals. Ruminant production systems contribute significantly to the CH4 emission (Ku-Vera et al., 2020). In dry land, CH4 can occur under anaerobic conditions (Lafuente et al., 2020). The formation of CH4 gas is closely associated with the activities of methanogen bacteria that require organic material and anaerobic environments. Therefore, the formation of CH4 in the study sites can be said to have been caused by anaerobic condition with decomposing organic matter, which abetted methanogen bacterial activity (Jiang et al., 2019).
(Fig 3.)
Fig 3. shows that CH4 flux could be negative, indicating soil uptake of atmospheric CH4 (Werner et al., 2006). It could also be caused by bacterial (methanogen and methanotroph) activity. Methanogen bacteria activity on dry land is very limited; these bacteria can work only in narrow anaerobic sites with sufficient organic material. These sites might have formed during the initial gas sampling (time: 0 min) however, on subsequent sampling occasions (time: 20, 40 min), apparently the methanogens could not manufacture CH4 gas in the absence of suitable site conditions. Consequently, some measurable concentrations of CH4 gas could be detected in the early (0 min) measurement; but at the succeeding observations (20, 40 min), the CH4 concentrations did not increase, and even tended to fall, thereby causing the CH4 flux to become negative.
Besides methanogen (methane-forming) bacteria), there is also CH4 oxidizing (methanotroph) bacteria. Methanotroph bacteria is an aerobic microorganism that can grow and evolve with CH4 as the sole energy source (Ahmadi and Lackner, 2024). Thus, CH4 oxidation can occur in micro-aerobic environment in the root zone and in the oxic soil surface layer. CH4 oxidation process is initiated by methane mono-oxygenase enzyme that plays a role in the conversion of CH4 into methanol (Kumar et al., 2021). The formation of CH4 gas on dry land is very limited but the aerobic conditions might support methanotroph bacterial activity hence, the CH4 gas that
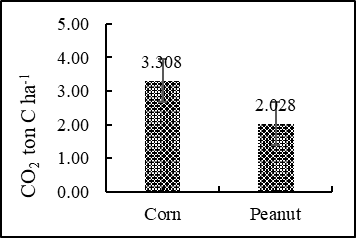
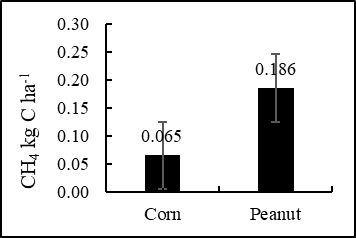
Fig 2. Total CO2 and CH4 fluxes from corn and peanut fields
formed at limited sites can be utilized by methanotroph bacteria (Guerrero-Cruz et al., 2021). This causes the CH4 gas concentrations to continue to decrease and lead to a negative flux value. Negative flux values in dry land farming had been found by previous researchers. For instance, flux in the soybean cultivation was 0.05 mg C-CH4 m-2 h-1 (Ernawanto et al., 2003). Methane fluxes were also low and negative (that is, CH4 uptake) in most cases and ranged on average from - 1.66 to - 1.22 mg m-2 day-1 in Mediterranean dry land (Lafuente et al., 2020).
Net Primary Production (NPP)
Carbon is fixed by the Earth’s vegetation, as NPP. NPP refers to the net content of organic matter synthesized by plants through the uptake of CO2 (photosynthesis) minus the consumption by plant autotrophic respiration per unit area and time (Chen et al. 2023). This is the carbon or biomass yield of the landscape, available for use by animals and humans. Our estimates of NPP do not include
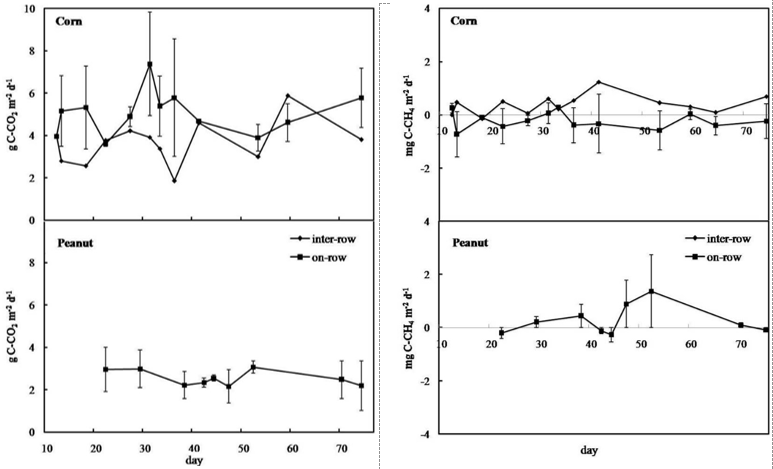
Fig 3. Cumulative carbon dioxide (CO2) and methane (CH4) fluxes.
carbon in root exudates. If elevated CO2 significantly increased root exudation, then our estimates of NPP in each plot would have been too low. Interestingly, some studies have found no significant increases in root exudation in plants grown under elevated CO2 (Doughty et al. 2018). In this study, the NPP value of each land during a cropping season that was obtained reached 5.50 ton C ha-1 and 2.71 ton C ha-1 in the corn and peanut fields, respectively. The summary of plant carbon values in each field are presented in Fig 3.
NPP serves as an indicator of the rise in plant biomass, which is a crucial aspect of the global carbon cycle and provides insight into the well-being of an ecosystem (Yelin Jiang et al., 2020). (Kirschbaum et al., 2001) cited that NPP constitutes the total annual growth increment (both above and below ground) plus the amount grown and shed in senescence, reproduction or death of short-lived individuals in a stand plus the amount consumed by herbivores.
The analysis of plant carbon content yielded the following calculated NPP values for one growing season: roughly 5.50 ton C ha-1 in the corn field and 2.71 ton C ha-1 in the peanut field. The comparison of the corn and peanut NPP was carried out with the key assumption that each field was planted successively with the same crop for one year. In other words, given the respective length of each crop growing season, each field was cultivated and planted to corn or peanut about 4 times per year. With this assumption, the NPP value for each area would amount to around 21.98 ton C ha-1 yr-1 for corn, and 10.86 ton C ha-1 yr-1, for the peanut, field. These results give the estimated amount of carbon stored by plant during growth (biomass) in a field, which also provide estimates of the amount of CO2 in the atmosphere that is absorbed by plants.
Based on calculated values for one year, it can be seen that the highest emission came from the corn land, together with the higher value of NPP. This suggests that the higher the carbon that is released through respiration
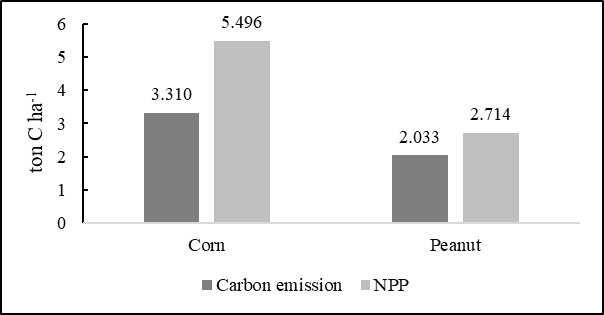
Fig 4. The amount of carbon emissions and NPP in each area.
by a farmland, the higher the carbon that is absorbed through photosynthesis. This is in accordance with (Jarvi and Burton, 2020) who reported the amount of the amount of total C that released back to the atmosphere affected by root respiration. Conversely, (Collalti et al., 2020) stated that respiration is not linearly related either to photosynthesis or to biomass, but it is more strongly controlled by recent photosynthates (and reserve availability) than by total biomass.
Soil ecosystems act as absorbers, reservoirs and emitters of GHG, depending on the balance of inputs and outputs, which are conditioned by different processes that influence GHG emissions such as soil biological respiration, rate of nitrification and other oxidative processes including soil erosion and land use change (Muñoz et al., 2010). Strategies to mitigate carbon dioxide emissions through changing in management practices that has the potential to enhance the forest carbon balance and reduce emissions (Law et al., 2018). In China and the United States have shown that changes in farm management with reduce chemical fertilizer use can reduce GHG emissions, increases in yields might also be achieved through the adoption of agroecological production practices, including cover crops, integrated pest management, and increased use of precision agriculture, but will require different management interventions in different regions (Clark et al., 2020).
NPP values indicate the amount of carbon contained during the period of plant growth. In this regard, we recommend that after harvesting the remnants of the plant crop should not be removed or burned. Farmers should return the remaining plant materials back into the soil to increase soil organic matter, which can help improve the physical condition and fertility of the soil. Along this line, (Amelung et al., 2020) suggested that improving capabilities in management practices to the soil, can retain soil at high organic carbon input, irrespective of which form this carbon exists and how it is stabilized. This results in increased infiltration, better soil water relations, reduced surface sealing and erosion which should lead to increased crop yields. The improvement and maintenance of soil C and soil structure are essential for sustainable agricultural systems and conservation of soil resources.
Materials and methods
Site description
The research was conducted in the Ranca Bungur District of Bogor, West Java, Indonesia, on land that had traditionally been utilized for conventional agriculture, specifically for cultivating corn and peanut crops. The research carried on eight months from November to June and included continuous monitoring of soil CO2 and CH4 fluxes. Plant samples for the assessment of Net Primary Production (NPP) were collected at harvest: corn at 77 days after planting and peanuts at 75 days after planting. For the sampling, four corn plants and nine peanut plants were selected for analysis. Additionally, plant samples were collected for the assessment of NPP. CO2 gas and plant sample analyses were performed at the laboratory of the Department of Soil Science and Land Resources, Faculty of Agriculture, IPB University, Indonesia. The analysis of CH4 gas samples was conducted at the Laboratory of Soil Science, Graduate School of Agriculture, Hokkaido University, Japan.
The soil at the research site is classified as Aquic Dystrudept, and the climate is tropical. In Indonesia, the seasons are generally divided into the dry season and the rainy season. There is a significant temporal variation in CO2 emissions, which is closely related to climatic factors such as temperature, humidity, rainfall, and the distribution of precipitation within a region. The distinct conditions during the dry season differ markedly from those during the rainy season, leading to substantial influences on CO2 emissions throughout the year. A comparative experimental design was employed, focusing on two types of land use: corn and peanuts, with plots located in close proximity to ensure uniform environmental and soil conditions. Standard agricultural practices, including fertilization and irrigation, were implemented across all plots.
Carbon flux measurements
The observation period coincided with the cropping time (from planting to harvesting) for corn (Zea mays) and peanut (Arachis hypogaea), namely: 77 days and 75 days, respectively (Kuswandora, 2012a). The recommended standard method for GHG flux measurements are closed chamber method (Pavelka et al., 2018). It involves creating a closed space on the soil surface and measuring CO2 concentration in the inner space (Bekku et al., 1995). The model of the closed chamber method can be seen in Figure 1. The CO2 and CH4 fluxes were taken weekly by closed chamber method, two replications on row, one replication inter-row in corn, and three replications in peanut field (without row). Flux estimates were based on changes in chamber CO2 and CH4 concentrations over time. Four gas samples (at 0-, 3-, 6- and 12- min intervals) were injected into tedlar bags while three gas samples (at 0-, 20- and 40- min intervals) were injected into vial bottles. CO2 and CH4 fluxes were measured using CO2 infrared gas analyzer and gas chromatography with flame ionization detector (FID).
Samples of corn (Zea mays L.) and peanut (Arachis hypogaea L.) were likewise taken when harvested. All parts of the plant were taken to measure the plant carbon content using a CHNS elemental analyzer. For corn, the plant samples consisted of roots, stems, leaves, flowers, and fruits; while for peanut, the sample consisted of both above-ground parts (leaves, stems and flowers) and underground ground parts (roots and pods). Plant samples were collected at four replicates in corn and nine replicates in peanut fields. Each plant sample was weighed and then oven-dried at 600C for 72 hours. After drying, samples were cut into small sections, crushed and then sieved with a 100-mesh stainless steel screen. Afterwards, total C and N content were analyzed using CHNS elemental autoanalyzer. To determine water content and biomass, the plant samples were oven-dried at 1000C for 24 hours (or until its weight reached a more or less fixed level.
Net Primary Production (NPP) calculation
Plant carbon measurements were conducted at harvest for each field. All parts of the plant samples were collected for carbon content analysis. For corn, samples included roots, stems, leaves, flowers, and ears. In contrast, peanut samples comprised the above-ground parts (leaves, stems, and flowers) and below-ground parts (roots and pods). After collecting representative plant samples, the weight of each sample was recorded, followed by oven drying at 60°C for 72 hours. Once dried, the samples were chopped and ground, then filtered through a 100 mesh sieve. Carbon content analysis was performed using a CHNS elemental analyzer. Additionally, the moisture content and dry weight of the plant samples (biomass) were determined by further oven drying at 1000°C for 24 hours until a constant weight was achieved.
Carbon content analysis is commonly used to determine the amount of
NPP. NPP represents the amount of carbon that is incorporated into
biomass, and is the difference between total carbon assimilated by
photosynthesis (gross primary production or GPP) and that portion lost
by autotrophic respiration (Ra) (Sierra, Estupinan-Suarez and
Chanca, 2021). Therefore, predicting the effects of elevated
CO2 on NPP under different environmental conditions requires
a deep understanding of the effects of CO2 on GPP and
Ra. For example, an increase in NPP could be driven by the
increased fixation of carbon into the system (increased GPP), or reduced
flux of carbon out of the system (reduced Ra), or both. In this
research, we obtained the amount of NPP during the crop growing period
(77 days for corn and 75 days for peanut) by multiplying the total plant
biomass at harvest in one hectare with carbon content. The NPP
calculation formula used was:
NPP = B x C
where:
NPP is Net Primary Production (ton C ha-1 period-1),
B as the biomass (t ha-1period-1), and
C is carbon content (% C)
Only the amount of carbon produced and lost in the year for which NPP is being calculated was counted, not what was produced in an earlier year and lost in the current year. However, in practice, this distinction is sometimes difficult to make.
Conclusion
The total soil carbon emissions from the corn and peanut fields were 3.310 and 2.033 ton C ha-1, respectively. This study also found that CH4 flux rates were very low, and oxidative processes dominated over production. Meanwhile, the Net Primary Production (NPP) values were found to be 21.98 ton C ha-1 yr-1 for corn and 10.86 ton C ha-1 yr-1 for peanut. The positive relationship between carbon emissions and NPP suggests that an increase in NPP could be driven by the increased fixation of carbon into the system or reduced flux of carbon out of the system, or both. Overall, the study confirmed that CO2 flux is strongly influenced by the respiration of plant roots and the high activity levels of microorganisms around the roots.
Reference
Ahmadi F, Lackner M (2024) Recent findings in methanotrophs: genetics, molecular ecology, and biopotential. Appl Microbiol Biotechnol. 108:60. https://doi.org/10.1007/s00253-023-12978-3
Amelung W, Bossio D, de Vries W, Kögel-Knabner I, Lehmann J, Amundson R, Bol R, Collins C, Lal R, Leifeld J, Minasny B, Pan G, Paustian K, Rumpel C, Sanderman J, van Groenigen JW, Mooney S, van Wesemael B, Wander M, Chabbi A (2020) Towards a global-scale soil climate mitigation strategy. Nat Commun 11:5427. https://doi.org/10.1038/s41467-020-18887-7
Badewa EA, Yeung CC, Rezanezhad F, Whalen JK, Oelbermann M (2022) Spring Freeze–Thaw Stimulates Greenhouse Gas Emissions From Agricultural Soil. Front Environ Sci 10
Bekku Y, Koizumi H, Nakadai T, Iwaki H (1995) Measurement of soil respiration using closed chamber method: An IRGA technique. Ecol Res 10:369–373. https://doi.org/10.1007/BF02347863
Bernoux M, Branca G, Carro A, Lipper L, Smith G, Bockel L (2010) Ex-ante greenhouse gas balance of agriculture and forestry development programs. Sci Agric 67:31–40. https://doi.org/10.1590/S0103-90162010000100005
Chapin FS, Matson PA, Vitousek PM (2011) Carbon Inputs to Ecosystems. In: Chapin FS, Matson PA, Vitousek PM (eds) Principles of Terrestrial Ecosystem Ecology. Springer, New York, NY, pp 123–156
Chen S, Zhao W, Zhang R, Sun X, Zhou Y, Liu L (2023) Higher Sensitivity of NIRv,Rad in Detecting Net Primary Productivity of C4 Than that of C3: Evidence from Ground Measurements of Wheat and Maize. Remote Sens 15:1133. https://doi.org/10.3390/rs15041133
Chuai X, Yuan Y, Zhang X, Guo X, Zhang X, Xie F, Zhao R, Li J (2019) Multiangle land use-linked carbon balance examination in Nanjing City, China. Land Use Policy 84:305–315. https://doi.org/10.1016/j.landusepol.2019.03.003
Clark MA, Domingo NGG, Colgan K, Thakrar SK, Tilman D, Lynch J, Azevedo IL, Hill JD (2020) Global food system emissions could preclude achieving the 1.5° and 2°C climate change targets. Science 370:705–708. https://doi.org/10.1126/science.aba7357
Collalti A, Tjoelker MG, Hoch G, Mäkelä A, Guidolotti G, Heskel M, Petit G, Ryan MG, Battipaglia G, Matteucci G, Prentice IC (2020) Plant respiration: Controlled by photosynthesis or biomass? Glob Change Biol 26:1739–1753. https://doi.org/10.1111/gcb.14857
Doughty CE, Goldsmith GR, Raab N, Girardin CAJ, Farfan-Amezquita F, Huaraca-Huasco W, Silva-Espejo JE, Araujo-Murakami A, da Costa ACL, Rocha W, Galbraith D, Meir P, Metcalfe DB, Malhi Y (2018) What controls variation in carbon use efficiency among Amazonian tropical forests? Biotropica 50:16–25. https://doi.org/10.1111/btp.12504
Ernawanto Q, Saein M, Sastiono A, Partohardjono S (2003) Dinamika metana pada lahan sawah tadah hujan dengan pengolahan tanah, varietas, dan bahan organik yang berbeda. Forum Pascasarj IPB 26:241–255
Fan M, Li Q, Zhang E, Liu Q, Wang Q (2019) Effects of mulching on soil CO2 fluxes, hay yield and nutritional yield in a forage maize field in Northwest China. Sci Rep 9:14186. https://doi.org/10.1038/s41598-019-50475-8
FAO (2011) Mainstreaming Carbon Balance Appraisal in Agriculture. EX-ACT: A Tool to Measure the Carbon-Balance: issue Papers. EASYPol Module 099. FAO, Rome, Italy
Feng C, Sun Z, Zhang L, Feng L, Zheng J, Bai W, Gu C, Wang Q, Xu Z, van der Werf W (2021) Maize/peanut intercropping increases land productivity: A meta-analysis. Field Crops Res 270:108208. https://doi.org/10.1016/j.fcr.2021.108208
Feng H, Guo J, Han M, Wang W, Peng C, Jin J, Song X, Yu S (2020) A review of the mechanisms and controlling factors of methane dynamics in forest ecosystems. For Ecol Manag 455:117702. https://doi.org/10.1016/j.foreco.2019.117702
Gao B, Huang T, Ju X, Gu B, Huang W, Xu L, Rees RM, Powlson DS, Smith P, Cui S (2018) Chinese cropping systems are a net source of greenhouse gases despite soil carbon sequestration. Glob Change Biol 24:5590–5606. https://doi.org/10.1111/gcb.14425
Ghosh D, Mandal A (2023) Climate Change: Its Impact on Land Degradation and Plant Nutrients Dynamics. In: Pande CB, Moharir KN, Negm A (eds) Climate Change Impacts in India. Springer International Publishing, Cham, pp 189–209
Guerrero-Cruz S, Vaksmaa A, Horn MA, Niemann H, Pijuan M, Ho A (2021) Methanotrophs: Discoveries, Environmental Relevance, and a Perspective on Current and Future Applications. Front Microbiol 12. https://doi.org/10.3389/fmicb.2021.678057
Heimsch L, Lohila A, Tuovinen J-P, Vekuri H, Heinonsalo J, Nevalainen O, Korkiakoski M, Liski J, Laurila T, Kulmala L (2021) Carbon dioxide fluxes and carbon balance of an agricultural grassland in southern Finland. Biogeosciences 18:3467–3483. https://doi.org/10.5194/bg-18-3467-2021
Hendri J, Sumawinata B, Baskoro DPT (2015) CO2 Flux from Tropical Land Uses on Andisol in West Java, Indonesia. J Trop Soils 19:121–130. https://doi.org/10.5400/jts.2014.v19i3.121-130
IPCC (2007) Summary for Policymakers. In: Climate Change 2007: The Physical Science Basis. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change [Solomon, S., D. Qin, M. Manning, Z. Chen, M. Marquis, K.B. Averyt, M.Tignor and H.L. Miller (eds.)]. Cambridge University Press, Cambridge, UK, United Kingdom
IPCC (1997) Climate Change 1995: Impacts, Adaptations and Mitigation of Climate Change: Scientific-Technical Analyses. [Robert T. Watson, M.C. Zinyowera, and Richard H. Moss (eds)]. Cambridge University Press, Cambridge, UK
IPCC (ed) (2022) Land degradation. In: Climate Change and Land: IPCC Special Report on Climate Change, Desertification, Land Degradation, Sustainable Land Management, Food Security, and Greenhouse Gas Fluxes in Terrestrial Ecosystems. Cambridge University Press, Cambridge, pp 345–436
Jarvi MP, Burton AJ (2020) Root respiration and biomass responses to experimental soil warming vary with root diameter and soil depth. Plant Soil 451:435–446. https://doi.org/10.1007/s11104-020-04540-1
Jia S, Liang A, Zhang S, Chen X, McLaughlin NB, Sun B, Zhang X, Wu D (2021) Effect of tillage system on soil CO2 flux, soil microbial community and maize (Zea mays L.) yield. Geoderma 384:114813. https://doi.org/10.1016/j.geoderma.2020.114813
Jiang J, Wang Y, Liu J, Yang X, Ren Y, Miao H, Pan Y, Lv J, Yan G, Ding L, Li Y (2019) Exploring the mechanisms of organic matter degradation and methane emission during sewage sludge composting with added vesuvianite: Insights into the prediction of microbial metabolic function and enzymatic activity. Bioresour Technol 286:121397. https://doi.org/10.1016/j.biortech.2019.121397
Jiang Y, Guo J, Peng Q, Guan Y, Zhang Y, Zhang R (2020a) The effects of climate factors and human activities on net primary productivity in Xinjiang. Int J Biometeorol 64:765–777. https://doi.org/10.1007/s00484-020-01866-4
Jiang Y, Zhang B, Wang W, Li B, Wu Z, Chu C (2020b) Topography and plant community structure contribute to spatial heterogeneity of soil respiration in a subtropical forest. Sci Total Environ 733:139287. https://doi.org/10.1016/j.scitotenv.2020.139287
Kaiser MS (2020) Land Degradation: Causes, Impacts, and Interlinks with the Sustainable Development Goals. In: Leal Filho W, Azul AM, Brandli L, Özuyar PG, Wall T (eds) Responsible Consumption and Production. Springer International Publishing, Cham, pp 1–13
Kirschbaum M, Eamus D, Gifford R, Roxburgh S, Sands P (2001) Definitions Of Some Ecological Terms Commonly Used In Carbon Accounting
Kumar M, Yadav AN, Saxena R, Rai PK, Paul D, Tomar RS (2021) Novel methanotrophic and methanogenic bacterial communities from diverse ecosystems and their impact on environment. Biocatal Agric Biotechnol 33:102005. https://doi.org/10.1016/j.bcab.2021.102005
Kuswandora VD (2012a) Emisi Gas CO2 dan Neraca Karbon pada Lahan Jagung, Kacang Tanah, dan Singkong di Kecamatan Ranca Bungur, Bogor. Institut Pertanian Bogor
Kuswandora VD (2012b) Emisi Gas CO2 dan Neraca Karbon pada Lahan Jagung, Kacang Tanah, dan Singkong di Kecamatan Ranca Bungur, Bogor. IPB (Bogor Agricultural University)
Ku-Vera JC, Jiménez-Ocampo R, Valencia-Salazar SS, Montoya-Flores MD, Molina-Botero IC, Arango J, Gómez-Bravo CA, Aguilar-Pérez CF, Solorio-Sánchez FJ (2020) Role of Secondary Plant Metabolites on Enteric Methane Mitigation in Ruminants. Front Vet Sci 7
Lafuente A, Durán J, Delgado-Baquerizo M, Recio J, Gallardo A, Singh BK, Maestre FT (2020) Biocrusts Modulate Responses of Nitrous Oxide and Methane Soil Fluxes to Simulated Climate Change in a Mediterranean Dryland. Ecosystems 23:1690–1701. https://doi.org/10.1007/s10021-020-00497-5
Law BE, Hudiburg TW, Berner LT, Kent JJ, Buotte PC, Harmon ME (2018) Land use strategies to mitigate climate change in carbon dense temperate forests. Proc Natl Acad Sci 115:3663–3668. https://doi.org/10.1073/pnas.1720064115
Li Z, Zhang Z, Lin C, Chen Y, Wen A, Fang F (2016) Soil–air greenhouse gas fluxes influenced by farming practices in reservoir drawdown area: A case at the Three Gorges Reservoir in China. J Environ Manage 181:64–73. https://doi.org/10.1016/j.jenvman.2016.05.080
Manzoni S, Čapek P, Porada P, Thurner M, Winterdahl M, Beer C, Brüchert V, Frouz J, Herrmann AM, Lindahl BD, Lyon SW, Šantrůčková H, Vico G, Way D (2018) Reviews and syntheses: Carbon use efficiency from organisms to ecosystems – definitions, theories, and empirical evidence. Biogeosciences 15:5929–5949. https://doi.org/10.5194/bg-15-5929-2018
Marlina ET, Kurnani TBA, Hidayati YA, Rahmah KN, Harlia E (2018) The potential of various livestock waste as sources of methanogenic bacteria. J Powder Technol Adv Funct Mater 1:19–23. https://doi.org/10.29253/jptafm.1.1.2018.3
Matthews ML (2023) Engineering photosynthesis, nature’s carbon capture machine. PLOS Biol 21:e3002183. https://doi.org/10.1371/journal.pbio.3002183
Medhi K, Bhardwaj R, Laxmi R (2021) Climate Change with Its Impacts on Soil and Soil Microbiome Regulating Biogeochemical Nutrient Transformations. In: Choudhary DK, Mishra A, Varma A (eds) Climate Change and the Microbiome: Sustenance of the Ecosphere. Springer International Publishing, Cham, pp 95–138
Mehra P, Baker J, Sojka RE, Bolan N, Desbiolles J, Kirkham MB, Ross C, Gupta R (2018) A Review of Tillage Practices and Their Potential to Impact the Soil Carbon Dynamics. In: Advances in Agronomy. Elsevier, pp 185–230
Muñoz C, Paulino L, Monreal C, Zagal E (2010) Greenhouse Gas (CO2 AND N2O) Emissions from Soils: A Review. Chil J Agric Res 70:485–497. https://doi.org/10.4067/S0718-58392010000300016
National Geographic Society (2023) Carbon Sources and Sinks
Nijbroek R, Piikki K, Söderström M, Kempen B, Turner KG, Hengari S, Mutua J (2018) Soil Organic Carbon Baselines for Land Degradation Neutrality: Map Accuracy and Cost Tradeoffs with Respect to Complexity in Otjozondjupa, Namibia. Sustainability 10:1610. https://doi.org/10.3390/su10051610
Nugroho PA, Shimizu M, Nakamato H, Nagatake A, Suwardi S, Sudadi U, Hatano R (2015) Nitrous oxide fluxes from soil under different crops and fertilizer management. Plant Soil Environ 61:385–392. https://doi.org/10.17221/164/2015-PSE
Nugroho PA, Sudadi U, Suwardi S (2018) Effect of Fertilizer Management on Soil Carbon Dioxide Fluxes in Grassland and Cornfield during Winter. J Agric Sci Technol 20:841–853
Pausch J, Kuzyakov Y (2018) Carbon input by roots into the soil: Quantification of rhizodeposition from root to ecosystem scale. Glob Change Biol 24:1–12. https://doi.org/10.1111/gcb.13850
Pavelka M, Acosta M, Kiese R, Altimir N, Brümmer C, Crill P, Darenova E, Fuß R, Gielen B, Graf A, Klemedtsson L, Lohila A, Longdoz B, Lindroth A, Nilsson M, Jiménez SM, Merbold L, Montagnani L, Peichl M, Pihlatie M, Pumpanen J, Ortiz PS, Silvennoinen H, Skiba U, Vestin P, Weslien P, Janous D, Kutsch W (2018) Standardisation of chamber technique for CO2, N2O and CH4 fluxes measurements from terrestrial ecosystems. Int Agrophysics 32:569–587. https://doi.org/10.1515/intag-2017-0045
Raich J, Schlesinger W (1992) The global carbon dioxide flux in soil respiration and its relationship to vegetation and climate. Tellus 44:81–99
Reicosky DC (1997) Tillage-induced CO2 emission from soil. Nutr Cycl Agroecosystems 49:273–285. https://doi.org/10.1023/A:1009766510274
Rochette P, Flanagan LB, Gregorich EG (1999) Separating Soil Respiration into Plant and Soil Components Using Analyses of the Natural Abundance of Carbon-13. Soil Sci Soc Am J 63:1207–1213. https://doi.org/10.2136/sssaj1999.6351207x
Rumbang N, Radjagukguk B, Prajitno D (2009) Emisi Karbon Dioksida (CO2) dari Beberapa Tipe Penggunaan Lahan Gambut di Kalimantan. J Ilmu Tanah Dan Lingkung 9:95–102
Ryusuke H, Suwardi, Bellingrath-Kimura SD (2015) Key processes and factors to mitigate land degradation. CATENA 133:453–454. https://doi.org/10.1016/j.catena.2015.07.011
Sharififar A, Minasny B, Arrouays D, Boulonne L, Chevallier T, van Deventer P, Field DJ, Gomez C, Jang H-J, Jeon S-H, Koch J, McBratney AB, Malone BP, Marchant BP, Martin MP, Monger C, Munera-Echeverri J-L, Padarian J, Pfeiffer M, Richer-de-Forges AC, Saby NPA, Singh K, Song X-D, Zamanian K, Zhang G-L, van Zijl G (2023) Chapter Four - Soil inorganic carbon, the other and equally important soil carbon pool: Distribution, controlling factors, and the impact of climate change. In: Sparks DL (ed) Advances in Agronomy. Academic Press, pp 165–231
Shukla SK, Khan A, Rao TS (2021) Chapter 22 - Microbial fouling in water treatment plants. In: Das S, Dash HR (eds) Microbial and Natural Macromolecules. Academic Press, pp 589–622
Sierra CA, Estupinan-Suarez LM, Chanca I (2021) The fate and transit time of carbon in a tropical forest. J Ecol 109:2845–2855. https://doi.org/10.1111/1365-2745.13723
Suprihati S, Anas I, Sabiham S, Djajakirana G (2006) Fluks Metana dan Karakteristik Tanah pada Beberapa Macam Sistem Budidaya. J Agron Indones 34:7957. https://doi.org/10.24831/jai.v34i3.1299
Warner DL, Bond-Lamberty B, Jian J, Stell E, Vargas R (2019) Spatial Predictions and Associated Uncertainty of Annual Soil Respiration at the Global Scale. Glob Biogeochem Cycles 33:1733–1745. https://doi.org/10.1029/2019GB006264
Werner C, Zheng X, Tang J, Xie B, Liu C, Kiese R, Butterbach-Bahl K (2006) N2O, CH4 and CO2 emissions from seasonal tropical rainforests and a rubber plantation in Southwest China. Plant Soil 289:335–353. https://doi.org/10.1007/s11104-006-9143-y
Whitman WB, Bowen TL, Boone DR (2006) The Methanogenic Bacteria. In: Dworkin M, Falkow S, Rosenberg E, Schleifer K-H, Stackebrandt E (eds) The Prokaryotes: Volume 3: Archaea. Bacteria: Firmicutes, Actinomycetes. Springer, New York, NY, pp 165–207
Zheng X, Guo J, Song W, Feng J, Lin G (2018) Methane Emission from Mangrove Wetland Soils Is Marginal but Can Be Stimulated Significantly by Anthropogenic Activities. Forests 9:738. https://doi.org/10.3390/f9120738