

Aust J Crop Sci. 19(04):332-341 (2025) | ISSN:1835-2707
https://doi.org/10.21475/ajcs.25.19.04.p223
From induction to embryo proliferation: improved somatic embryogenesis protocol in cork oak (Quercus suber L.)
Naouar Ben Ali*1, Zineb El Ansari Nejjar2, Nosair El Yakoubi1, Fouad Oumassi1, Mustapha Hassoun1, Loubna Benamar3, El Moussaoui Abdelfattah3, Loubna Bounab4, Brahim El Bouzdoudi1, Mohammed L’Bachir El Kbiach1
1Laboratory of Plant Biotechnology, Department of Biology, Faculty of Sciences of Tetouan, Abdelmalek Essaadi University, Tetouan, Morocco
2Life and Health Sciences Team, Faculty of Medicine and Pharmacy, Abdelmalek Essaadi, University, Tetouan, Morocco
3Laboratory of Ecology, Systematic and Conservation of Biodiversity (LESCB), URL-CNRST N° 18, Faculty of Sciences, Abdelmalek Essaadi Univercity, Tetouan, Morocco
4Advanced Materials, Structures and Civil Engineering Team, ENSA Tetouan, Abdelmalek Essaadi University, Tetouan 93000, Morocco
*Corresponding author: Naouar Ben Ali 
 ORCID:
https://orcid.org/0000-0002-1023-5589
ORCID:
https://orcid.org/0000-0002-1023-5589
Abstract: Cork oak (Quercus suber L.) is the dominant species in the forests ecosystems of Northern Morocco, comprising 72% of the area. It is an economically important species, producing high-quality cork, wood and acorns, and offers significant potential for further improvement. Somatic embryogenesis (SE) is the basis of clonal forestry for these species. One challenge with this approach is that SE induction in cork oak has not yet been fully optimized, especially the process of secondary somatic embryogenesis (SSE). Our study focused on developing a reproducible procedure for SSE to produce mature somatic embryos in cork oak. We evaluated the response of different PGR and physical parameters on the morphological development and somatic embryogenesis of cork oak. Darkness and a temperature of 25±2°C yielded the best results (93.30%). Additionally, incubation in complete darkness (in an oven for 2 months)) produced better responses than exposure to a 16-hour photoperiod provided by cool-white fluorescent lamps at a photon flux density of 50-60 µmol m-2s- 1. For secondary somatic embryo production, the use of Naphthalene Acetic Acid (NAA) at a concentration of 1mg/l in N30K medium blocked the germination process in cork oak somatic embryos. Regarding to secondary somatic embryogenesis, the use of ANA had no effect on the process. While the combination of ANA and BAP at 1mg/l showed better effect on stem and leaf formation (0.22 ±0.07abc) and (0.71± 0.25bc) respectively. No significant interaction with activated charcoal was observed. In this study, we describe the conditions required for studying the process of secondary somatic embryogenesis, including darkness, temperature and other key parameters. It was concluded that the N30K medium, a temperature of 25°C and full darkness produced the best results.
Key words: Quercus suber; somatic embryogenesis; somatic embryos; in vitro culture; auxin/cytokinin.
Abbreviations: SE_ Somatic embryogenesis; SSE _ secondary somatic embryogenesis; BAP_6-benzylaminopurine; NAA_ Naphthalene Acetic Acid; PEMs_ proembryogenic masses; PGRs_ Plant growth regulators; AC_Activated charcoal.
Introduction
Quercus suber L., known as cork oak, is an evergreen forest tree species naturally distributed in the western Mediterranean basin, occurring across a wide range of geographic and climatic conditions (Martinez et al., 2020). It is considered one of the most important Mediterranean forest tree species that can plays an important ecological role as environmental protector, as it is one of the key species in the Mediterranean ecosystem, contributing to soil conservation as well as the biodiversity of flora and fauna. Socially, cork oak plays a key role in in sustainable activities, providing a source of income for rural populations across the Mediterranean basin due to the economic value of cork (Capote et al., 2019).
In Morocco, cork oak ecosystems represent an important heritage, economic wealth and social asset of national importance. These forests cover approximately 377,482 hectares and extend across the north-western part of the country, from the coastal plains to the Central Rif and the Middle Atlas. The cork oak forests and woodlands play a determining role socially, economically and environmentally (El Antry and Piazzetta., 2014). However, as in other Southern countries, they face strong pressures and degradations.
The cork oak is highly threatened by several factors, such as forest fires and poor natural regeneration. However, the most important threat is (Corredoira et al., 2018) the effect of global warming, the intensification of illegal harvesting of forest resources, and the growing dynamics of urbanization, leisure and recreation dynamics (Banabou et al., 2022) which have caused a high mortality rate in the last years.
This is why the Moroccan authorities have undertaken an extensive artificial regeneration program for cork oak by

Figure 1. A general description is required first. A) Tree selected in the Maâmora region for taking cuttings; B-C) Sterilization and classification of cuttings by diameter, disbudding of cuttings and formation of epicormic shoots; D) Epicormic shoots formation.
planting. This effort has been accompanied by research into plant production and stand management. The establishment of plantations with genetically improved materials is desirable. Within the strategies of genetic improvement programs for oaks, vegetative propagation plays a main role (Corredoira et al., 2018). Current strategies of forest tree breeding emphasize on the use of vegetative propagation to rapidly capture all the potential of selected individuals and establish them in plantations, a process known as Multi-Varietal Forestry (Park et al., 2016).
The use of biotechnological methods, such as in vitro cloning and genetic transformation have also been reported as useful alternatives for woody plants (Corredoira et al., 2019).
Plant tissue culture is a promising tool for plant production and trait improvement, and has secured a significant position in biotechnology. It is based on the principle of totipotency, which is defined as the ability of any plant cell to convert into a whole plant when provided with the appropriate conditions of nourishment (Rai et al., 2022).
In the cases of forest tree improvement, somatic embryogenesis is a powerful system whose major applications are large-scale propagation of selected material, genetic transformation and cryopreservation of elite genotypes (Corredoira et al., 2017). Somatic embryos mainly originate from proembryogenic masses (PEMs), which are rounded/nodular structures of cellular aggregates that arise from leaf explants after induction, as the first morphological sign of embryogenic response (Corredoira et al., 2012). PEMs contain embryogenic cells that can give rise to somatic embryos or proliferate and produce more PEMs (Steiner et al., 2016). The factors controlling the process of somatic embryogenesis are the synchrony of somatic embryo development, maturation, and conversion of somatic embryos into plantlets (Garcia et al., 2019).
Somatic embryogenesis consists of four phases. The first stage is where explants (callus retaining cells with embryogenic competence) initiate development of vegetative cells or tissues. In the second stage, a group of cells destined for embryogenesis, known as embryogenic cell lines, are maintained and developed. The third stage involves the formation of somatic embryo (embryo undergoes globular, heart-shaped, torpedo, and cotyledonary stages) and maturation (accumulation of reserve substances). Finally, somatic embryos are converted (germinated) into viable plantlets (Rai et al., 2022).
Secondary somatic embryogenesis is a phenomenon in which a new somatic embryo is produced from a primary somatic embryo. Compared to the primary somatic embryogenesis, the secondary one offers several advantages, including a higher multiplication rate, independence of an explant source, and repeatability. Furthermore, embryogenicity can be maintained for prolonged periods by repeated cycles of the secondary embryogenesis. Secondary embryogenesis has been shown to have higher propagation efficiency than the primary somatic embryogenesis mentioned above (Raemakers et al., 1995).
However, this process is influenced by the effect of several phytohomonal parameters such as: effect of PGR as well as physical parameters such as: light effect, temperature effect, etc.
Plant growth regulators (PGRs) are chemical substances used in the plant tissue culture media for their specific function. These PGRs play a key role in organogenesis. There are different types of PGRs available in nature with specific functions, as well as synthetic PGRs added to artificial plant tissue culture media for organogenesis modifications of plant growth and development (Ramon et al., 2021). Auxins, cytokinins, gibberellins, abscisic acid, and ethylene are the major classes of PGRs. The ratio of auxin to cytokinins incorporated into the media defines the specific response concerning organogenesis, which is unique to a particular species (Saad and Elshahed, 2012).
Physical factors such as photoperiod and temperature also play a key role in the somatic embryogenesis process. The most important physical factor is light (Olarieta et al., 2019). Light exposure of a specific wavelength influences the particular developmental stages of SE (Rai et al., 2022). Light conditions play a significant role when working with somatic embryogenesis, as light contains spectrum of colors. The spectral distribution of colors in white fluorescent varies, whereas in LEDs it is specific. In addition to light, the duration of light and dark periods is also considered in many studies. Depending on the plant’s photoperiod, different light and dark hours, viz., 12h/12 h, 14h/10 h, 16 h/8h, and 24h light or dark, can be set for the experiment (Rai et al., 2022).
Most plant species respond well in 22°C-25°C. Some exceptional cases may be seen where increasing temperature increases the plant’s growth (Kaur and Mudgal, 2021). Lowering the temperature along with changing media composition is beneficial for the long-term storage of the plant material (Rai et al., 2022).
Activated charcoal (AC) is one of the essential components in plants tissue culture, often used in tissue culture to improve cell growth and development (Vitri Garvita and Sahromi, 2019). It plays an important role in somatic embryogenesis, rooting, stem elongation, seed germination and culturing of anthers and protoplasts. One of the most important functions of activated charcoal is reducing browning in the tissue culture medium (Thomas, 2008). This occurs because activated charcoal can release and/or adsorbs substances produced by cultures or present in the medium, which may promote and/or inhibit the in vitro growth of plants or explants (Vitri Garvita and Sahromi, 2019).
The aim of this study is to apply a somatic embryogenesis protocol for the clonal propagation of Moroccan cork oak, detailing all the steps of this technique from the starting material to the obtention of the somatic embryos. However, the main objective is the study of secondary somatic embryogenesis, such as one of the most important steps of the technique, in order to develop procedures for somatic embryo proliferation. This will enable selected embryogenic lines to be
Table 1. Effect of light (photoperiode for 16h) and full darkness for 24 h on secondary embryogenesis.
| Treatments | Average number of secondary embryos | Average number of clusters | Average number of embryo clusters |
|---|---|---|---|
| Light (photoperiod for 16-h) | 1.71 ± 0.39 | 0.31 ± 0.07 | 0.74 ± 0.19 |
| Full Darkness | 2.51 ± 0.29 | 0.37 ± 0.09 | 1.25 ± 0.33 |
Table 2. Influence of temperature on the induction of secondary somatic embryos (ES II) of cork oak after eight weeks in the dark.
| Temperature (°C) |
Number of primary reaction embryos | SSE (%) |
|---|---|---|
| 15 | 8 | 26.66 |
| 20 | 15 | 50.00 |
| 25 | 28 | 93.30 |
| 27 | 25 | 83.33 |
| 30 | 23 | 76.66 |
| 33 | 19 | 63.66 |
indefinitely maintained, as well as to define the germination process and plant recovery from somatic embryos for future reforestation program. In this context, we aim to investigate the effects of hormones and external factors on the somatic embryogenesis and the control of secondary embryogenesis in Moroccan cork oak.
Results
Figure 3 shows the first signs of somatic embryogenesis, after 130 days of culture.
Embryogenic response of embryos to light
Light quality can also influence embryogenesis, and initial explant cultures need to be maintained in darkness at 25°C for 6 weeks (Rathore et al., 2020; Corredoira et al., 2019). White light promotes plant growth, but also leads to an increased production of phenolic compounds in the medium. These materials induce oxidative reactions, causing the tissue to turn brown (Pintos et al., 2008). To avoid the effects of light, we tested the response of secondary embryos to light exposure (Table 1). According to these results, the incubation in the dark appears to be more favorable, but the statistical analysis shows that the difference between dark and light conditions is not very significant. Under light conditions, the embryos appear greenish, whereas they appear yellowish white in the dark (Fig 4). From a quantitative perspective, darkness seems to play a slightly more favorable role the secondary somatic embryogenesis process. Therefore, primary somatic embryos tend togrow better in the dark, producing more secondary embryos.
Effect of temperature to secondary somatic embryos formation
Many studies have demonstrated that somatic embryo development (induction, maturation, and germination) is sensitive to temperature variations. In our study, we tested the major effect of light on the process of secondary somatic embryogenesis. The results clearly show that temperature significantly influences the multiplication and regeneration of secondary somatic embryos. The process of secondary somatic embryogenesis appears to be inhibited at temperatures below 15°C and above 33°C. The maximum number of secondary embryos is formed at approximately 25°C (Fig. 5), which is the optimal temperature for the multiplication of cork oak embryos via secondary somatic embryogenesis. We also note that Duncan's post-hoc test reveals that the statistical difference between the results obtained at 25°C and 27°C is not significant.
The number of clusters formed appears to be less sensitive to temperature variation, but varies according to the same trend, reaching a maximum near 25°C. Similarly, although the number of embryo clusters is relatively low, but it is significantly affected by temperature changes.
We conclude that temperature has a significant effect on the development of secondary embryos. At critical temperatures (15°C and 33°C), we can clearly see that development of the embryos is very low and even almost no development occurring at temperatures below 15°C. On the other hand, if we increase temperature, we can clearly see that the formation of secondary embryos acts positively at temperature in the range of 25±2°C, C where the SSE process is very active (almost 6 secondary embryos were formed for each primary embryo) (Table 2). Regarding clusters, it was noticed, and for the first time, that temperature has little to no effect on their formation, even at lower temperatures compared to higher values. This suggests that the formation of cluster is more influenced by biotic factors than by climatic ones. Concerning the embryo clusters, the average number is relatively low since the clusters do not respond significantly to temperature changes, but for those that are already formed, they are influenced more or less by the effect of the temperature.
Effect of activated charcoal to SSE process
Activated charcoal is often used in tissue culture to improve cell growth and development. (Vitri Garvita et al., 2019). It is considered one of the essential components in tissue culture media due to its significant adsorptive properties on phenol and other growth inhibitory substances (Fernando et al., 2010). In our study, activated charcoal had no positive effect on secondary somatic embryogenesis. Figure 6 explain that the addition of activated charcoal (AC) to the medium show a significant decrease in the regeneration number of secondary somatic embryos, clusters and embryo clusters at all concentrations compared to the control medium.
Effect of auxins/cytokinins to secondary somatic embryogenesis and germination
Effect of auxin
The addition of phytohormones to the culture medium significantly reduced the number of secondary embryos, the average number of roots formed on secondary embryos, and the number of clusters formed on secondary embryos. Except for BAP at 1mg/L, which slightly increased the number of stems and leaves (0.07±0.03bc to 0.45±0.13a) and number ostems and leaves/embryo (0.31±0.17bc to 1.90±0.44a), although the difference was not statistically significantly (Fig. 9).
In this experiment, the use of NAA at a concentration of 1 mg/l blocked the germination process in cork oak somatic embryos, while its combination with BAP at 1 mg/l showed a slightly significant effect on stem and leaf formation (0.22 ±0.07abc) and (0.71± 0.25bc), respectively (Table 1).
Discussion
Light is one of the most important parameters for successful promising in vitro plant production (Miler et al., 2019). It plays a crucial role in influencing enzymes and genes activity, as well as the growth of explants (Lin et al., 2011; Azmi et al., 2016; Manivannan et al., 2017). The duration of the light and dark periods is considered in many studies.
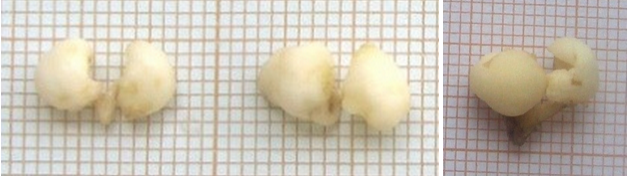
Figure 2. Primary somatic embryos: 3 mature embryos at the cotylidonary stage (8-10mm).
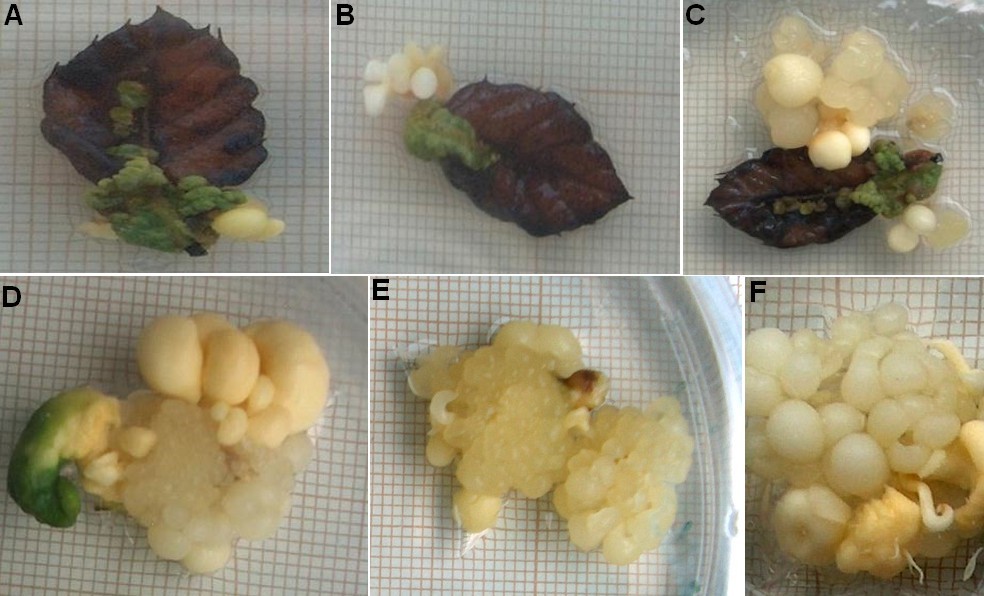
Figure 3. Somatic embryogenesis of Moroccan cork oak from leaves and proliferation process of secondary somatic embryogenesis. (A-C) Induction of primary somatic embryogenesis and embryo formation; (D) Proliferation of somatic embryos and formation of secondary somatic embryogenesis; (E, F) Neoformation of clusters.
According to plant photoperiodism, different light-dark hours may be set for the experiment. In our study, light had no significant effect on secondary somatic embryogenesis. This finding is consistent with the study of Li et al. (2019) which showed that in Acacia melanoxylon, the light quality, with varying ratio of red/blue light, had no significant effect on bud growth in vitro (Li et al., 2019).
However, light quality does affects somatic embryogenesis in Cydonia oblonga Mill. A positive effect of red light was observed, leading to an increase in somatic embryo production (D’Onofrio et al., 1998). In addition, light was found suitable for the in vitro growth of Cunninghmia lanceolate (Xu et al., 2020).
In the literature, it has been found that in Quercus ilex the percentage of secondary somatic embryos is significantly higher when somatic embryos are grown in the dark (Mauri and Manzanera, 2003). Furthermore, light had no significant effect on embryo germination. Some studies, including ours, have also shown that light can influence the quality of the somatic embryogenesis process, depending on the stage of the development of the embryos. Darkness should be maintained until the cotyledonary stage is reached, at which point exposure to light is recommended (Pinto et al., 2008).
Temperature plays a crucial role in somatic embryogenesis. It is a determining factor that influence the number and the morphology of embryogenic cell lines, as well as the biochemical profile of somatic embryos and the resulting somatic plants (Castander-Olarieta et al. 2019, 2021; Pereira et al., 2021).
Different stages in the process, such as embryogenic induction, embryo proliferation, and maturation can be significantly influenced by temperature. The induction of somatic embryogenesis was clearly influenced by temperature during secondary somatic embryogenesis.
There are few studies examining the effect of temperature on secondary SE formation and plant regeneration. The most
significant result of our investigation is that a relatively moderate temperature (25° C) is better for induction of secondary SE (Hua et al., 2010). In general, species respond well to temperatures between 22-25°C, although some exceptional cases have shown that higher temperatures can promote plant growth (Kaur and Mudgal, 2021). In other studies, increased temperature during somatic embryogenesis can induce earlier budburst in Abies nordmanniana (Lobo et al., 2022). In Hovenia dulcis, high temperature (30°C) was effective for induction of secondary somatic embryos, while in low temperature (20°C) were more suitable for further embryo development (Yang et al., 2013). In addition, in Pinus radiata and Pinus halepensis, the application of high temperatures during SE initiation influences the success of the different stages of the process.
In the previous studies, we observed that the application of high temperatures during the initiation of Pinus radiata and Pinus halepensis SE modulates not only the success of the different stages of the process, but also the morphology and biochemical status (hormones, metabolites) of embryonal masses and somatic embryos (Castander-Olarieta et al., 2021; Periera et al., 2020).
Activated Charcoal (AC) can help overcome the inhibitory effects of phenolic released into the vitro culture medium. It can release and/or adsorbs substances produced by the cultures or present in the medium, which promote and/or inhibit the in vitro development of plants or explants (Vitri Garvita and Sahromi, 2019). Most reports confirm the positive role of AC in the medium, promoting the development of plant tissues. In Quercus suber somatic embryos, the presence of activated charcoal did not promote repetitive embryogenesis, while in its absence, repetitive embryogenesis appeared at the base of many embryos (Thomas, 2008; Pintos et al., 2010), which is consistent with our study.
The most significant effect of adding AC to culture media is a drastic radical reduction in the concentration of PGRs and other organic supplements. This occurs due to the adsorption of these

Figure 4. Secondary somatic embryos formation of cork oak. A) Embryo formation under darkness; B, C) Embryo formation under light.
![C:\Users\Elegens\Desktop\[8]8 messages in conversationAJCS-Hassoun-PNE-223 [Major revisions required]\22-02\[8]8 messages in conversationAJCS-Hassoun-PNE-223 [Major revisions required]\Figure\Figure 5 F22.jpg](vertopal_3554b7712a9a4bbca1a3c6f87961d145/media/image8.jpeg)
Figure 5. Influence of temperature on secondary somatic embryogenesis of cork oak.
![C:\Users\Elegens\Desktop\[8]8 messages in conversationAJCS-Hassoun-PNE-223 [Major revisions required]\22-02\[8]8 messages in conversationAJCS-Hassoun-PNE-223 [Major revisions required]\Figure\Figure F22.jpg](vertopal_3554b7712a9a4bbca1a3c6f87961d145/media/image9.jpeg)
Figure 6. Influence of Activated Charcoal (AC) concentration on the induction of Secondary Somatic Embryogenesis (SSE) of cork oak.
chemicals by AC, which can leave researchers C unaware of the actual dose available to the plant tissues (Thomas, 2008). Another example of the negative effect of AC was reported in Myrciaria aureana somatic embryogenesis (Motoike et al., 2007) and Castanea dentate (Xing et al.,1999). However, many studies demonstrate the efficiency of AC in promoting advanced somatic embryogenesis in many species, including date palm (Ghazzawy et al., 2017), Pinus pinaster, P. sylvestris (Germana and Lambardi, 2016), Begum jungi (Solanji et al., 2023) and saffron (Chib et al., 2020).
Cytokinin promotes the advanced induction of somatic embryos, germination, and shoots formation. BAP has been noted to stimulate somatic embryo germination and shoot development in Carica papaya L. (Solórzano-Cascante et al., 2018). Moreover, optimal PGR contents have only been determined for some cultivars or genotypes (Martínez and Corredoira, 2023). A combination of BAP and NAA is effective in
inducing somatic embryos in Corydalis yanhusuo (Sagare et al., 2000, Rai et al., 2022).
Plant media supplementary additives, such phytohormones as PGRs, amino acids, antioxidants, etc., alone or in combination can effectively influence the regeneration process. Many studies in various plant species have highlighted the auxin/cytokinin interaction as a key factor in promoting plant regeneration (Raspor et al., 2021).
The combination of NAA and BAP has also been used to induce somatic embryos from leaf tissues in other Fagaceae species, including oaks, where embryogenic cultures have been initiated from both juvenile (Cuenca et al., 1999), adult and leaf explants (Hernández et al., 2003; Toribio et al. 2004; Martinez et al., 2015; Pérez et al., 2015a and b). However, in our case, this combination had no effect on our genotype.
In Quercus robur, the use of a germination medium containing BAP produced better results than a medium without growth
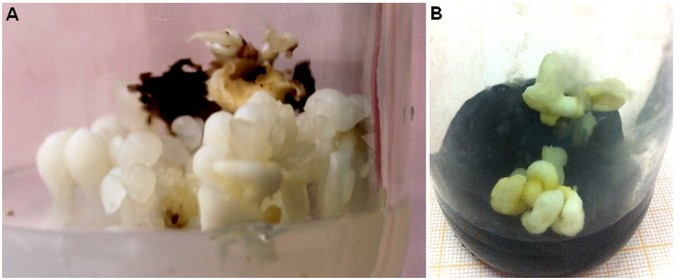
Figure 7. Proliferation of somatic embryos of Quercus suber L. A) Control medium showing embryo differentiation; B) Proliferated embryos after tow month on culture medium with charcoal.
![C:\Users\Elegens\Desktop\[8]8 messages in conversationAJCS-Hassoun-PNE-223 [Major revisions required]\22-02\[8]8 messages in conversationAJCS-Hassoun-PNE-223 [Major revisions required]\Figure\Figure 8 F22.jpg](vertopal_3554b7712a9a4bbca1a3c6f87961d145/media/image11.jpeg)
Figure 8. Effect of auxin/cytokinins on secondary somatic embryogenesis of cork oak.
regulators (Vieitez et al, 2012). Similarly, in Quercus bicolor, better results were obtained by adding BAP at 0.25 µM or 0.44 µM to the culture medium (Mallon et al., 2013). In Eucalyptus. Globules, the proliferation of globular SE increased by reducing the NAA level, as well as in a medium devoid of PGRs (Pinto et al., 2008; Yelli et al., 2023).
This discrepancy in the results obtained for different species may be due to the origin of the explant used and the genotype (Mallon et al., 2013). Concerning the germination protocol, the genotype influences the germinative capacity of somatic embryos in several species. In Quercus suber, 40 to 70% of embryos germinated, depending on the maturation treatment and the genotype (Toribio et al., 2005, Ben Ali et al., 2023).
Media without or containing low concentrations of phytohormones, such as cytokinins (BAP), have often shown promising results, with germination rates of approximately 90% (Junairiah et al., 2019; Yelli et al., 2023; Wilhelm, 2000). In other studies, the presence of both auxin and cytokinin in the medium promotedthe formation of new meristematic primordia and prevented their differentiation into roots (Raspor et al., 2021).
Materials and methods
Here we report the most important steps of somatic embryogenesis in cork oak, following the outline: Plant materials, Induction of Primary Somatic Embryogenesis, Secondary Somatic Embryogenesis.
Plant materials and culture conditions
The starting explants (cuttings, 2-3 cm in length and 2 cm in diameter) were isolated from branches segments selected from the elite trees located in the Maâmora region (Morocco: GPS: N: 34°03’029, w: 006°38’207). The cuttings were sterilized, and classified according to their diameters, and cultured to obtain epicormic shoots.
Induction of primary somatic embryogenesis
Young apical leaves, approximately 0.5 cm in size, excised from epicormic shoots were used as explants to induce primary somatic embryogenesis. The collected young leaf explants were thoroughly rinsed under running tap water, surface sterilized with 70% ethanol for 30 s, then stirred in an aqueous solution of calcium hypochlorite (65% active chlorine) at 7%, with 2 drops of Tween 80 /100 ml, for about 20 min. They were then disinfected with HgCl2 0.1 % (w/v) for 5 min and washed three times with sterile water to remove any traces of HgCl2. The sterilized leaves were placed in Petri dish with preconditioning medium (Hernandez, 2007, Ben Ali et al., 2023).

Figure 9. Germination of secondary embryos of cork oak: effect of auxin/cytokinin.
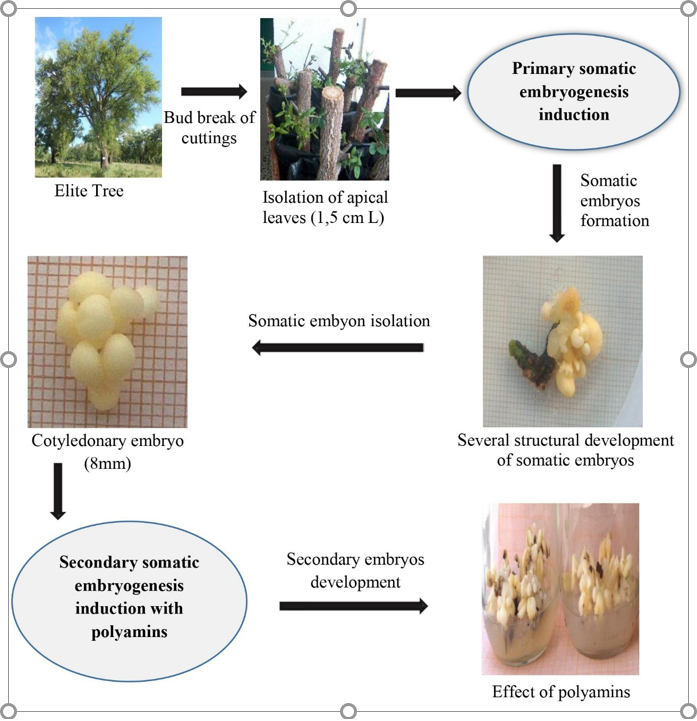
Figure 10. Induction of primary and secondary somatic embryos of cork oak: a summary of steps.
The cultures were incubated under controlled culture room condition of temperature (25± 2°C) and darkness for 8 days. After 7 days, the leaves were transferred to the primary induction medium in darkness for 30 days. The second transfer was to the secondary induction medium under a photoperiod condition for 30 days, before being transferred to a proliferation medium under the same conditions.
Induction of secondary somatic embryogenesis
For secondary somatic embryogenesis induction, primary mature embryos (8-10 mm in length and at the cotyledonary stage) (Fig.2) were solely initiated from leaves explants and were reported to be embryogenic. Many experiments were conducted to evaluate the potential for the embryonic proliferation of somatic embryos.
Culture conditions
In all experiments, embryogenic cultures were proliferated through repetitive embryogenesis with subculturing at 8-week intervals in Petri dishes (9 cm diameter) containing 20 mL of multiplication medium. This medium consisted of N30K macronutrients (Margara, 1978), mineral salts, Murashige and Skoog (MS) vitamins (Murashige and Skoog, 1962), 3% sucrose (w/v) and 0.7% plant propagation agar (bacteriological agar type E). After adjusting the pH to 5.6, the medium was autoclaved at 120°C for 20 min. The embryogenic cultures were incubated in the dark (in an oven in full darkness for 2 months) at 25 ± 2 C°, the darkness here was considered as control condition for all the experiments before testing its effect. For each treatment, at least 30 explants were used, and all experiments were repeated three times.
Effect of light
Two sets of experiments were performed to evaluate the effect of light. In the first experiment, embryos were exposed to illumination with a 16-hour photoperiod (provided by cool-white fluorescent lamps at a photon flux density of 50-60 µmol m⁻²s⁻¹) for 8 weeks.
In the second experiment, embryos were kept under dark conditions (incubated in the dark (full darkness for 24 hours) all the 8 weeks.
Culture conditions: All initial explant cultures were maintained at 25°C for 8 weeks.
Number of embryos: Each treatment involved 30 embryos.
Repetitions: The experiments were repeated three times.
Effect of temperature
The mature embryos were cultured in proliferation medium poured into Petri dishes and incubated in the dark. For the temperature effect, the experimental design, protocol and methodology regarding the embryo treatment can be sumarized as follow:
Number of embryos per temperature: For each temperature (15, 20, 25, 27, 30, and 33°C), 180 embryos were cultured.
Repetitions of the experiment: The experiment was repeated three times, meaning that for each temperature, 60 embryos were used per repetition (60 x 3 = 180).
Total number of embryos: In total, 540 embryos were used across all temperature tests (180 embryos per temperature x 3 repetitions = 540).
Effect of auxins/cytokinins
Experiments were performed using somatic embryos cultured medium. The first experiment tested three concentrations of BAP (0.5, 1, and 2 mg/L). The second experiment used 1 mg/L of NAA in the same medium. The third experiment combined NAA and BAP at 1 mg/L each.
Number of embryos per temperature: For each temperature (15, 20, 25, 27, 30, and 33°C), 180 embryos were cultured.
Repetitions of the experiment: The experiment was repeated three times, meaning that for each temperature, 60 embryos were used per repetition (60 x 3 = 180).
Total number of embryos: In total, 540 embryos were used across all temperature tests (180 embryos per temperature x 3 repetitions = 540).
Effect of activated charcoal
The experimental design for the activated charcoal treatment can be summarized as follow: Culture conditions: Somatic embryos were placed into flask culture cells, with two embryos per flask, containing 40 ml of N30K culture media (Margara, 1978) with various concentrations of activated charcoal (0, 0.3, 0.5, and 1 mg/L), 30% sucrose, 8% agar, MS (Murashige and Skoog 1962) micronutrients and vitamins, 0.7% bacteriological agar (type E), and 100 mg/L myo-inositol.
Embryogenic lines: These were established from somatic embryos derived from cork oak leaves. Secondary somatic embryogenesis was induced and expressed by maintaining cultures on N30K proliferation medium supplemented with the different concentrations of activated charcoal (0, 0.3, 0.5 and 1 mg/L).
pH and sterilization: The pH was adjusted to 5.8 before autoclaving at 120°C for 20 minutes.
Incubation: Cultures were incubated at 25 ± 2°C for two months in the dark.
Repetitions: Each experiment was conducted with three replications for statistical analysis.
Statistical analysis
Each experimental factor was tested independently. Therefore, a one-way ANOVA was applied separately for each factor (e.g., light, charcoal, hormones). All values obtained from the mean replicates were averaged. The data were analyzed in relation to the variance and presented as mean ± standard error (SE). The data were statistically analyzed using one-way ANOVA, by the statistical software SPSS 17.0 (2019). One-way analysis of variance (ANOVA) was carried out to determine differences between the treatments that produced cotyledonary somatic embryos. Multiple comparisons were made using Duncan’s post-hoc test (p ≤0.05).
Conclusion
The most challenging aspect of new plant breeding programs is the development of indirect regeneration protocols, as each genotype requires a specific regeneration protocol, especially for recalcitrant woody species.
In this work, we reported the necessary conditions for an optimal control of the secondary somatic embryogenesis in cork oak (Fig.10). Secondary somatic embryogenesis is a process in which a new somatic embryo is produced from a primary somatic embryo. Compared to the primary somatic embryogenesis, secondary somatic embryogenesis offers advantages such as higher multiplication rates, independence of an explant source, and repeatability. Furthermore, embryogenicity can be maintained for extended periods through repeated cycles of secondary embryogenesis.
The protocol established in this study will aid in the conservation and large scale vegetative propagation of these species. Controlling secondary embryogenesis is crucial, and performing a comparative investigation of histological development and genetic metabolites at each phase is essential for a better understanding of the regeneration process.
References
Ajbilou R, Marañón T, Arroyo J, Ater M (2007) Structure et diversité de la strate arbustive des forêts de la Péninsule Tingitane (Maroc). Acta Bot Malacit. 32: 147-160.
Azmi NS, Ahmad R, Ibrahim R (2016) Fluorescent light (FL), red led and blue led spectrums effects on in vitro shoots multiplication. J Teknol. 78: 93-97. https://doi.org/10.11113/jt.v78.9032
Banabou A, El Aboudi A, Ouareg Z, Abderrahmane AAFI, Chkhichekh A, Moukrim S, Laaribya S (2022) Classification binaire sur image satellitale multibandes pour la cartographie du recouvrement arboré (chêne-liège de la Maamora). Territ Environ Dév. 1(1): 1-12.
Ben Ali N, Benkaddour R, Rahmouni S, Boussaoudi I, Hamdoun O, Hassoun M, Azaroual L, Badoc A, Martin P, Lamarti A (2023) Secondary somatic embryogenesis in Cork oak: influence of plant growth regulators. For Sci Technol. doi: https://doi.org/10.1080/21580103.2023.2172462
Castander-Olarieta A, Montalba IA, De Medeiros Oliveira E, Dell’Aversana E, D’Amelia L, Carillo P, Steiner N, Fraga HPF, Guerra MP, Goicoa T, et al (2019) Effect of thermal stress on tissue ultrastructure and metabolite profiles during initiation of radiata pine somatic embryogenesis. Front Plant Sci. 9: 2004. doi.10.3389/fpls.2018.02004
Castander-Olarieta A, Pereira C, Montalbán IA, Mendes VM, Correia S, Suárez-Álvarez S, Manadas B, Canhoto J, Moncaleán P (2021) Proteome-wide analysis of heat-stress in Pinus radiata somatic embryos reveals a combined response of sugar metabolism and translational regulation mechanisms. Front Plant Sci. 12: 631239. doi:10.3389/fpls.2021.631239.
Chib S, Thangaraj A, Kaul S, Dhar MK, Kaul T (2020) Development of a system for efficient callus production, somatic embryogenesis and gene editing using CRISPR/Cas9 in Saffron (Crocus sativus L.). Plant Methods. 16(1): 1-10. https://doi.org/10.1186/s13007-020-00589-2.
Corredoira E, Merkle SA, Martínez MT, Toribio M, Canhoto JM, Correia SI, Ballester A, Vieitez AM (2019) Non-Zygotic Embryogenesis in hardwood species. Critical Reviews in Plant Sci. 38:1, 29-97, DOI: 10.1080/07352689.2018.1551122
Cuenca B, San-José C, Martínez MT, Ballester A, Vieitez AM (1999) Somatic embryogenesis from stem and leaf explants of Quercus robur L. Plant Cell Rep. 18:538-543.
D’Onofrio C, Morini S, Bellocchi G (1998) Effect of light quality on somatic embryogenesis of quince leaves. Plant Cell Tiss Org Cult. 53: 91-98.
El Antry S, Piazzetta R (2014) Les techniques de régénération du chêne-liège au Maroc. Forêt Méditerranéenne, For. Méditerr. XXXV (2): 161-170. https://hal.archives-ouvertes.fr/hal-03556645.
Garcia C, Almeida AAF, Costa M, Britto D, Valle R, Royaert S, Marelli JP (2019) Abnormalities in somatic embryogenesis caused by 2,4-D: an overview. Plant Cell Tiss Org Cult. 137: 193-212.
Germana MA, Lambardi M (Eds.) (2016) In vitro embryogenesis in higher plants. New York/Heidelberg: Humana Press. pp: 559.
Ghazzawy HS, Alhajhoj MR., Munir M (2017) In vitro somatic embryogenesis response of date palm cv. Sukkary to sucrose and activated characoal concentrations. J Appl Hortic. 19 (12): 91-95.
Hernandez I, Celestino C, Alegro J, Toribio M (2003) Vegetative propagation of Quercus suber L. by somatic embryogenesis: II. Plant regeneration from selected cork oak trees. Plant Cell Rep, 21: 765-770.
Hernández SI (2007) Regeneración clonal de alcornoques adultos (Quercus suber L.) mediante embriogénesis somática. Thèse de doctoral, pp: 1-241.
Hua YW, Huang TD and Huang HS (2010) Micropropagation of self-rooting juvenile clones by secondary somatic embryogenesis in Hevea brasiliensis. Plant Breed. 129: 202-207. doi:10.1111/j.1439-0523.2009.01663.x .
Junairiah J, Amalia NS, Manuhara YSW, Nimatuzahroh N, Sulistyorini L (2019) The effect of variation of growth regulating substances, IAA, BAP, Kinetin on secondary metabolites callus black belt (Piper betle L. Var Nigra). Jurnal Kim Ris. 4(2): 121-132.
https://doi.org/10.20473/jkr.v4i2.16898
Kaur J, Mudgal G (2021) An Efficient and Quick Protocol for In Vitro Multiplication of Snake Plant, Sansevieria trifasciata var. maurentii [Prain]. Plant Cell Tiss Org Cult. https://doi.org/10.21203/rs.3.rs-204936/v1.
Li S, Zhou L, Wu S Liu L, Huang M, Lin S, Ding G (2019) Effects of LED light on Acacia melanoxylon bud proliferation in vitro and root growth ex vitro. Open Life Sci. 14 (1): 349-357.In: Advances in plant tissue culture: Current developments and future trends (ed): Rai A., Modia A and Singh M, Elsevier. 2022, pp. 1-416.
Lobo A, Find JI, Hansen JK, Ræbild A, Kjær ED (2022) Effect of temperature and osmotic stress during somatic embryogenesis on phenology and physiology of abies nordmanniana emblings. For Ecol Manag. 514: 120-212.
Mallòn R, Martinez T, Corredoira E, Vieitez AM (2013) The positive effect of arabinogalactan on induction of somatic embryogenesis in Quercus bicolor followed by embryo maturation and plant regeneration. Trees. 27 (5): 1285-1296. https://doi.org/10.1007/s00468-013-0877-x
Manivannan A, Soundararajan P, Park YG, Wei H, Kim SH, Jeong BR (2017) Blue and red light-emitting diodes improve the growth and physiology of in vitro grown carnations ‘Green Beauty’ and ‘Purple Beauty’. Hortic Environ Biotechnol. 58: 12-20. https://doi.org/10.1007/s13580-017-0051-2
Margara J (1978) Mise au point d’une gamme de milieux mineraux pour les conditions de la culture «in vitro». C R. Seances Acad. Agric. Fr 64:654-661.
Martinez MT, Vieitez AM, Corredoira E (2015) Improved secondary embryo production in Quercus alba and Q. rubra by activated charcoal, silver thiosulphate and sucrose: influence of embryogenic explant used for subculture. Plant Cell Tiss Org Cult. 121 (3): 531-546. https://doi.org/10.1007/s11240-015-0722-6
Martínez MT and Corredoira E (2023) Efficient procedure for induction somatic embryogenesis in holm oak: Roles of explant type, auxin type, and exposure duration to auxin. Forests. 14: 430. https://doi.org/10.3390/f14020430
Martínez, MT, Vieitez FJ, Solla A, Tapias R, Ramírez-Martín N and Corredoira E (2020) Vegetative propagation of Phytophthora cinnamomi tolerant holm oak genotypes by axillary budding and somatic embryogenesis. Forest. 11: 841. https://doi.org/10.3390/f11080841
Miler N, Kulus D, Woźny A, Rymarz D, Hajzer M, Wierzbowski K, Nelke R, Szeffs L (2019) Application of wide-spectrum light-emitting diodes in micropropagation of popular ornamental plant species: a study on plant quality and cost reduction. In Vitro Cellular & Developmental Biology-Plant. 55: 99-108 (2019). https://doi.org/10.1007/s11627-018-9939-5.
Murashige T, Skoog F (1962) A revised medium for rapid growth and bio assays with Tobacco tissue culture. Physiol Plant. 15(3):473-497.
Capote T, Usié A, Barbosa P, Ramos M, Morais-Cecílio L, Gonçalves S (2019) Transcriptome dynamics of cork oak (Quercus suber) somatic embryogenesis reveals active gene players in transcription regulation and phytohormone homeostasis of embryo development. Tree Genet Genomes. 15:1-20. https://doi.org/10.1007/s11295-019-1353-6
Corredoira E, Hernández I, Morcillo M, Martínez MT, Ruiz-Galea M, Cernadas MJ, Ramírez-Martín N, San José MC, Arrillaga I, Toribio M (2018) Holm Oak Quercus ilex L. Protocoles par étapes pour l’embryogenèse somatique des plantes ligneuses importantes, Volume I: 181-195.
Fernando SC, Vidhanaarachchi VRM, Weerekoon LK Santha ES (2010) What makes clonal propagation of coconut difficult?. Asia-Pac J Mol Biol Biotechnol. 18(1): 163-165.
Motoike SY, Saraiva ES, Ventrella MC, Silva CV, Salomao LCC (2007) Somatic embryogenesis of Myrciaria aureana (Brazilian grape tree). Plant Cell Tiss Org Cult. 89: 75-81.
Castander-Olarieta A, Pereira C, Montalbán IA, Pencík A, Petrík I, Pavlovic I, Novák O, Strnad M, Moncaleán P (2020) Quantification of endogenous aromatic cytokinins in Pinus radiata embryonal masses after application of heat stress during initiation of somatic embryogenesis. Trees 35: 1075-1080. https://doi.org/10.1007/s00468-020-02047-x
Corredoira E, Hernández I, Morcillo M, Martínez MT, Ruiz-Galea M, Cernadas MJ, Ramírez-Martín N, San José MC, Arrillaga I, Toribio M (2018) Holm Oak Quercus ilex L. Protocoles par étapes pour l’embryogenèse somatique des plantes ligneuses importantes, Volume I: 181-195.
Corredoira E, Cano V, Bárány I, Solís MT, Héctor Rodríguez H, Vieitez AM, Risueno MC, Testillano PS (2017) Initiation of leaf somatic embryogenesis involves high pectin esterification, auxin accumulation and DNA demethylation in Quercus alba. J Plant Physiol. 213: 42-54.
Corredoira E, San-Jose MC, Vieitez AM (2012) Induction of somatic embryogenesis from different explants of shoot cultures derived from young Quercus alba trees. Trees-Struct. Funct. 26 (3): 881-891.
Lin Y, Li J, Li B, He T, Chun Z (2011) Effects of light quality on growth and development of protocorm - like bodies of Dendrobium officinale in vitro. Plant Cell Tiss Org Cult. 105: 329-335. https://doi.org/10.1007/s11240-010-9871-9
Mauri PV, Manzanera JA (2003) Induction, development and maturation of holm oak (Quercus ilex L.) somatic embryos. Plant Cell Tiss. Org. Cult. 74: 229-235.
Olarieta AC, Montalbán IA, Oliveira EDM, Dell’Aversana E, D’Amelia L, Carillo P, Steiner N, Fraga, HPDF, Guerra MP, Goicoa T, Ugarte MD, Pereira C, Moncaleán P (2019) Effect of thermal stress on tissue ultrastructure and metabolite profiles during initiation of Radiata Pine somatic embryogenesis. Front Plant Sci. 9: 1-16.
Pereira C, Castander-Olarieta A, Montalbán IA, Pencík A, Petrík I, Pavlovic I, De Medeiros Oliveira E, Fraga HPDF, Guerra MP, Novák O (2020) Embryonal masses induced at high temperatures in Aleppo pine: Cytokinin profile and cytological characterization. Forests. 11: 807.
Pereira C, Montalbán IA, Pedrosa A; Tavares J, Pestryakov A, Bogdanchikova N, Canhoto J, Moncaleán P (2021) Regeneration of Pinus halepensis (Mill.) through organogenesis from apical shoot buds. Forests. 12: 363.
Pérez M, Canal MJ, Toorop PE (2015) b. Expression analysis of epigenetic and abscisic acid-related genes during maturation of Quercus suber somatic embryos. Plant Cell Tiss Org Cult. 121 (2): 353-366. https://doi.org/10.1007/s11240-014-0706-y
Pérez M, Viejo M, La Cuesta M, Toorop P, Canal MJ (2015) Epigenetic and hormonal profile during maturation of Quercus suber L. somatic embryos. J Plant Physiol. 173: 51-61. https://doi.org/10.1016/j.jplph.2014.07.028
Park Y, Beaulieu J, Bousquet J (2016) Multi-varietal forestry integrating genomic selection and somatic embryogenesis. Veg Propag For Trees. 202-222.
Pinto G, Park YS, Silva S, Neves L, Araújo C, Santos C (2008) Factors affecting maintenance, proliferation, and germination of secondary somatic embryos of Eucalyptus globules Labill. Plant Cell Tiss Org Cult. 95: 69-78. https://doi.org/10.1007/s11240-008-9417-6
Pintos B, Bueno MA, Cuenca B, Manzanera JA (2008) Synthetic seed production from encapsulated somatic embryos of cork oak (Quercus suber L.) and automated growth monitoring. Plant Cell Tiss. Org. Cult. 95: 217-225.
Pintos B, Manzanera JA, Bueno MA (2010) Oak somatic and gametic embryos maturation is affected by charcoal and specific aminoacids mixture. Ann For Sci. 67: 205. doi: 10.1051/forest/2009098
Raemakers CJJM, Jacobsen E, Visser RGF (1995) Secondary somatic embryogenesis and applications in plant breeding. Euphytica. 81: 93-107.
Rai AC, Kumar A, Modi A, Singh M. (Eds.) (2022) Advances in Plant Tissue Culture: Current Developments and Future Trends. Academic Press. pp:1-409.
Ramon U, Weiss D, Illouz-Eliaz N (2021) Underground gibberellin activity: differential gibberellin response in tomato shoots and roots. New Phytol. 229: 1196-1200. https://doi.org/10.1111/nph.16876.
Raspor M, Motyka V, Kaleri AR, Ninković S, Tubić L, Cingel A (2021) Integrating the roles for cytokinin and auxin in de novo shoot organogenesis: from hormone uptake to signaling outputs. Int J Mol Sci. 22: 8554.
Rathore MS, Patel PR, Siddiqui SA (2020) Callus culture and plantlet regeneration in date palm (Phoneix dactylifera L.): an important horticultural cash crop for arid and semi-arid horticulture. Physiol Mol Biol Plants. 26: 391-398. https://doi.org/10.1007/s12298-019-00733-w
Saad AIM, Elshahed AM (2012) Plant Tissue Culture Media. Recent Adv. Plant Vitr Cult. 30-40.
Sagare A, Lee Y, Lin T, Chen C and Tsay H (2000) Cytokinin-induced somatic embryogenesis and plant regeneration in Corydalis yanhusuo (Fumariaceae)-a medicinal plant. Plant Sci. 160: 139-147.
Solangi N, Jatoi MA, Abul-Soad AA, Mirani AA, Solangi MA, Markhand GS, 2023. Factors influencing somatic embryogenesis and plantlet regeneration of date palm using immature floral buds. Sarhad J Agric. 39(2): 323-331.
Solórzano-Cascante P, Sánchez-Chiang N, Jiménez VM (2018) Explant type, culture system, 6-Benzyladenine, meta-topolin and encapsulation affect indirect somatic embryogenesis and regeneration in Carica papaya L. Front Plant Sci. 9: 1769.
Steiner N, Farias-Soares FL, Schmidt EC, Pereira ML, Scheid B, Rogge-Renner GD, Bouzon ZL, Schmidt D, Maldonado S, Guerra MP (2016) Toward establishing a morphological and ultrastructural characterization of proembryogenic masses and early somatic embryos of Araucaria angustifolia (Bert.) O. Kuntze. Protoplasma. 253 (2): 487-501.
Thomas, TD (2008) The role of activated charcoal in plant tissue culture. Biotechnol Adv. 26: 618-631.
Toribio M, Celestino C, Molinas M (2005) Cork oak, Quercus suber L. In: Jain S. M., Gupta P. K. (eds.) Protocol of somatic embryogenesis in woody plants. Forestry Sciences, 77
(35): 445-457. Springer, ISBN 1-4020-2984-5. https://doi.org/10.1007/1-4020-2985-3_35
Toribio M, Fernandez C, Celestino C, Martinez MT, San-Jose MC, Vieitez AM (2004) Somatic embryogenesis in mature Quercus robur trees. Plant Cell Tiss Org Cult. 76: 283-287
Vieitez AM, Corredoira E, Martínez MT, San-José MC, Sánchez C, Valladares S (2012) Application of biotechnological tools to Quercus improvement. Eur J For Res. 131: 519-539. https://doi.org/10.1007/s10342-011-0526-0
Vitri Garvita R, Sahromi (2019) Plant regeneration through direct somatic embryogenesis from leaf explants of Paraphalaenopsis Labukensis P. S. Shim. IOP Conf. Ser.: Earth Environ. Sci, 394: 012053.
Wilhelm E (2000) Somatic embryogenesis in oak (Quercus spp.). In Vitro Cell Dev Biol Plant. 36: 349-357. https://doi.org/10.1007/s11627-000-0062-y
Xing Z, Powell WA, Maynard CA (1999) Development and germination of American chestnut somatic embryos. Plant Cell Tiss Org Cult. 57:47-55.
Xu Y, Yang M, Cheng F, Liu S and Liang Y (2020) Effects of LED photoperiods and light qualities on in vitro growth and chlorophyll fluorescence of Cunninghamia lanceolate. BMC Plant Biol. 20:269. https://doi.org/10.1186/s12870-020-02480-7
Yang J, Wu S, Li CH (2013) High efficiency secondary somatic embryogenesis in Hovenia dulcis Thunb. through solid and liquid cultures. The Scientific World Journal. (2013), Article ID 718754, 6 pages. http://dx.doi.org/10.1155/2013/718754
Yelli F, Titin A, Utomo SD, Pathak A (2023) Somatic embryogenesis in two cassava (Manihot esculenta Crantz) genotypes. Not Bot Horti Agrobot. Cluj-Napoca. 51(1);13039. DOI:10.15835/nbha51113039.